Page 83 - Read Online
P. 83
Page 260 Thomas et al. J Transl Genet Genom 2024;8:249-77 https://dx.doi.org/10.20517/jtgg.2024.15
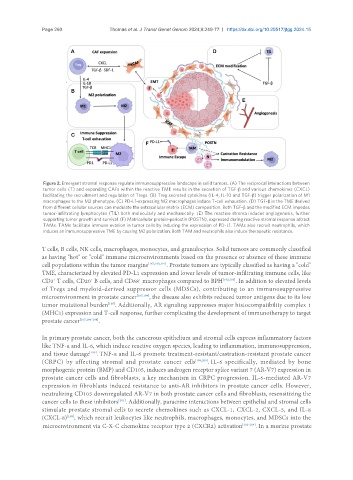
Figure 2. Emergent stromal response regulate immunosuppressive landscape in solid tumors. (A) The reciprocal interactions between
tumor cells (T) and expanding CAFs within the reactive TME results in the secretion of TGF-β and various chemokines (CXCL)
facilitating the recruitment and regulation of Tregs. (B) Treg secreted cytokines (IL-4, IL-10 and TGF-β) trigger polarization of M1
macrophages to the M2 phenotype. (C) PD-L1-expressing M2 macrophages induce T-cell exhaustion. (D) TGF-β in the TME derived
from different cellular sources can modulate the extracellular matrix (ECM) composition. Both TGF-β and the modified ECM impedes
tumor-infiltrating lymphocytes (TIL) both molecularly and mechanically. (E) The reactive stroma induces angiogenesis, further
supporting tumor growth and survival. (F) Matricellular protein-periostin (POSTN), expressed during reactive stromal response attract
TAMs. TAMs facilitate immune evasion in tumor cells by inducing the expression of PD-L1. TAMs also recruit neutrophils, which
induces an immunosuppressive TME by causing M2 polarization. Both TAM and neutrophils also induce therapeutic resistance.
T cells, B cells, NK cells, macrophages, monocytes, and granulocytes. Solid tumors are commonly classified
as having "hot" or "cold" immune microenvironments based on the presence or absence of these immune
cell populations within the tumor margins [167,190,191] . Prostate tumors are typically classified as having a "cold"
TME, characterized by elevated PD-L1 expression and lower levels of tumor-infiltrating immune cells, like
CD3 T cells, CD20 B cells, and CD68 macrophages compared to BPH [192,193] . In addition to elevated levels
+
+
+
of Tregs and myeloid-derived suppressor cells (MDSCs), contributing to an immunosuppressive
microenvironment in prostate cancer [167,194] , the disease also exhibits reduced tumor antigens due to its low
tumor mutational burden . Additionally, AR signaling suppresses major histocompatibility complex 1
[195]
(MHC1) expression and T-cell response, further complicating the development of immunotherapy to target
prostate cancer [167,196-198] .
In primary prostate cancer, both the cancerous epithelium and stromal cells express inflammatory factors
like TNF-α and IL-6, which induce reactive oxygen species, leading to inflammation, immunosuppression,
and tissue damage . TNF-α and IL-6 promote treatment-resistant/castration-resistant prostate cancer
[199]
(CRPC) by affecting stromal and prostate cancer cells [199,200] . IL-6 specifically, mediated by bone
morphogenic protein (BMP) and CD105, induces androgen receptor splice variant 7 (AR-V7) expression in
prostate cancer cells and fibroblasts, a key mechanism in CRPC progression. IL-6-mediated AR-V7
expression in fibroblasts induced resistance to anti-AR inhibitors in prostate cancer cells. However,
neutralizing CD105 downregulated AR-V7 in both prostate cancer cells and fibroblasts, resensitizing the
[201]
cancer cells to these inhibitors . Additionally, paracrine interactions between epithelial and stromal cells
stimulate prostate stromal cells to secrete chemokines such as CXCL-1, CXCL-2, CXCL-3, and IL-8
(CXCL-8) , which recruit leukocytes like neutrophils, macrophages, monocytes, and MDSCs into the
[199]
microenvironment via C-X-C chemokine receptor type 2 (CXCR2) activation [202-204] . In a murine prostate