Page 87 - Read Online
P. 87
Page 264 Thomas et al. J Transl Genet Genom 2024;8:249-77 https://dx.doi.org/10.20517/jtgg.2024.15
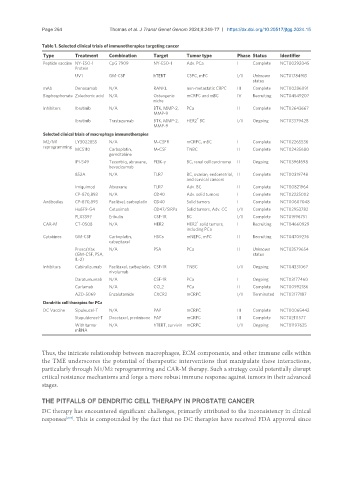
Table 1. Selected clinical trials of immunotherapies targeting cancer
Type Treatment Combination Target Tumor type Phase Status Identifier
Peptide vaccine NY-ESO-1 CpG 7909 NY-ESO-1 Adv. PCa I Complete NCT00292045
Protein
UV1 GM-CSF hTERT CSPC, mPC I/II Unknown NCT01784913
status
mAb Denosumab N/A RANKL non-metastatic CRPC III Complete NCT00286091
Bisphosphonate Zoledronic acid N/A Osteogenic mCRPC and mBC IV Recruiting NCT04549207
niche
Inhibitors Ibrutinib N/A BTK, MMP-2, PCa II Complete NCT02643667
MMP-9
+
Ibrutinib Trastuzumab BTK, MMP-2, HER2 BC I/II Ongoing NCT03379428
MMP-9
Selected clinical trials of macrophage immunotherapies
M2/M1 LY3022855 N/A M-CSFR mCRPC, mBC I Complete NCT02265536
reprogramming
MCS110 Carboplatin, M-CSF TNBC II Complete NCT02435680
gemcitabine
IPI-549 Tecentriq, abraxane, PI3K-γ BC, renal cell carcinoma II Ongoing NCT03961698
bevacizumab
852A N/A TLR7 BC, ovarian, endometrial, II Complete NCT00319748
and cervical cancers
Imiquimod Abraxane TLR7 Adv. BC II Complete NCT00821964
CP-870,893 N/A CD40 Adv. solid tumors I Complete NCT02225002
Antibodies CP-870,893 Paclitxel, carboplatin CD40 Solid tumors I Complete NCT00607048
Hu5F9-G4 Cetuximab CD47/SIRPa Solid tumors, Adv. CC I/II Complete NCT02953782
PLX3397 Eribulin CSF-1R BC I/II Complete NCT01596751
+
CAR-M CT-0508 N/A HER2 HER2 solid tumors, I Recruiting NCT04660929
including PCa
Cytokines GM-CSF Carboplatin, HSCs mNEPC, mPC II Recruiting NCT04709276
cabazitaxel
ProscaVax N/A PSA PCa II Unknown NCT03579654
(GM-CSF, PSA, status
IL-2)
Inhibitors Cabiralizumab Paclitaxel, carboplatin, CSF-1R TNBC I/II Ongoing NCT04331067
nivolumab
Daratumumab N/A CSF-1R PCa I Ongoing NCT03177460
Carlumab N/A CCL2 PCa II Complete NCT00992186
AZD-5069 Enzalutamide CXCR2 mCRPC I/II Terminated NCT03177187
Dendritic cell therapies for PCa
DC Vaccine Sipuleucel-T N/A PAP mCRPC III Complete NCT00065442
Stapuldencel-T Docetaxel, prednisone PAP mCRPC III Complete NCT02111577
With tumor N/A hTERT, survivin mCRPC I/II Ongoing NCT01197625
mRNA
Thus, the intricate relationship between macrophages, ECM components, and other immune cells within
the TME underscores the potential of therapeutic interventions that manipulate these interactions,
particularly through M1/M2 reprogramming and CAR-M therapy. Such a strategy could potentially disrupt
critical resistance mechanisms and forge a more robust immune response against tumors in their advanced
stages.
THE PITFALLS OF DENDRITIC CELL THERAPY IN PROSTATE CANCER
DC therapy has encountered significant challenges, primarily attributed to the inconsistency in clinical
[278]
responses . This is compounded by the fact that no DC therapies have received FDA approval since