Page 110 - Read Online
P. 110
Chu et al. J Transl Genet Genom 2023;7:196-212 https://dx.doi.org/10.20517/jtgg.2023.22 Page 104
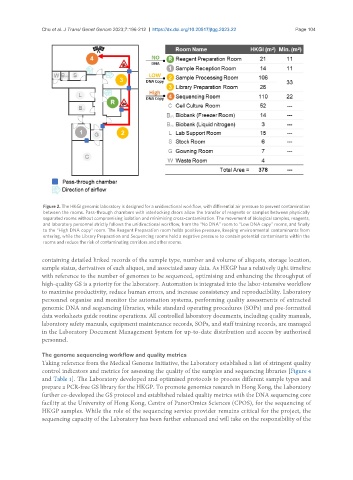
Figure 2. The HKGI genomic laboratory is designed for a unidirectional workflow, with differential air pressure to prevent contamination
between the rooms. Pass-through chambers with interlocking doors allow the transfer of reagents or samples between physically
separated rooms without compromising isolation and minimising cross-contamination. The movement of biological samples, reagents,
and laboratory personnel strictly follows the unidirectional workflow, from the “No DNA” room to “Low DNA copy” rooms, and finally
to the “High DNA copy” room. The Reagent Preparation room holds positive pressure, keeping environmental contaminants from
entering, while the Library Preparation and Sequencing rooms hold a negative pressure to contain potential contaminants within the
rooms and reduce the risk of contaminating corridors and other rooms.
containing detailed linked records of the sample type, number and volume of aliquots, storage location,
sample status, derivatives of each aliquot, and associated assay data. As HKGP has a relatively tight timeline
with reference to the number of genomes to be sequenced, optimising and enhancing the throughput of
high-quality GS is a priority for the laboratory. Automation is integrated into the labor-intensive workflow
to maximise productivity, reduce human errors, and increase consistency and reproducibility. Laboratory
personnel organise and monitor the automation systems, performing quality assessments of extracted
genomic DNA and sequencing libraries, while standard operating procedures (SOPs) and pre-formatted
data worksheets guide routine operations. All controlled laboratory documents, including quality manuals,
laboratory safety manuals, equipment maintenance records, SOPs, and staff training records, are managed
in the Laboratory Document Management System for up-to-date distribution and access by authorised
personnel.
The genome sequencing workflow and quality metrics
Taking reference from the Medical Genome Initiative, the Laboratory established a list of stringent quality
control indicators and metrics for assessing the quality of the samples and sequencing libraries [Figure 4
and Table 1]. The Laboratory developed and optimised protocols to process different sample types and
prepare a PCR-free GS library for the HKGP. To promote genomics research in Hong Kong, the Laboratory
further co-developed the GS protocol and established related quality metrics with the DNA sequencing core
facility at the University of Hong Kong, Centre of PanorOmics Sciences (CPOS), for the sequencing of
HKGP samples. While the role of the sequencing service provider remains critical for the project, the
sequencing capacity of the Laboratory has been further enhanced and will take on the responsibility of the