Page 110 - Read Online
P. 110
Schofield et al. J Cancer Metastasis Treat 2020;6:10 I http://dx.doi.org/10.20517/2394-4722.2019.43 Page 5 of 12
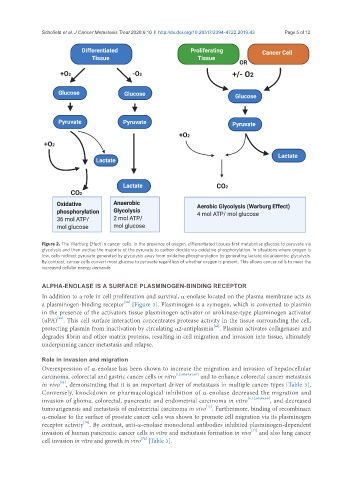
Figure 2. The Warburg Effect in cancer cells. In the presence of oxygen, differentiated tissues first metabolise glucose to pyruvate via
glycolysis and then oxidise the majority of the pyruvate to carbon dioxide via oxidative phosphorylation. In situations where oxygen is
low, cells redirect pyruvate generated by glycolysis away from oxidative phosphorylation by generating lactate via anaerobic glycolysis.
By contrast, cancer cells convert most glucose to pyruvate regardless of whether oxygen is present. This allows cancer cells to meet the
increased cellular energy demands
ALPHA-ENOLASE IS A SURFACE PLASMINOGEN-BINDING RECEPTOR
In addition to a role in cell proliferation and survival, a-enolase located on the plasma membrane acts as
[55]
a plasminogen-binding receptor [Figure 3]. Plasminogen is a zymogen, which is converted to plasmin
in the presence of the activators tissue plasminogen activator or urokinase-type plasminogen activator
[65]
(uPA) . This cell surface interaction concentrates protease activity in the tissue surrounding the cell,
protecting plasmin from inactivation by circulating a2-antiplasmin . Plasmin activates collagenases and
[66]
degrades fibrin and other matrix proteins, resulting in cell migration and invasion into tissue, ultimately
underpinning cancer metastasis and relapse.
Role in invasion and migration
Overexpression of a-enolase has been shown to increase the migration and invasion of hepatocellular
carcinoma, colorectal and gastric cancer cells in vitro [11,58,60,61,67] and to enhance colorectal cancer metastasis
[11]
in vivo , demonstrating that it is an important driver of metastasis in multiple cancer types [Table 3].
Conversely, knockdown or pharmacological inhibition of a-enolase decreased the migration and
invasion of glioma, colorectal, pancreatic and endometrial carcinoma in vitro [6,12,63,68,69] , and decreased
tumourigenesis and metastasis of endometrial carcinoma in vivo . Furthermore, binding of recombinant
[12]
a-enolase to the surface of prostate cancer cells was shown to promote cell migration via its plasminogen
[70]
receptor activity . By contrast, anti-a-enolase monoclonal antibodies inhibited plasminogen-dependent
[71]
invasion of human pancreatic cancer cells in vitro and metastasis formation in vivo and also lung cancer
[72]
cell invasion in vitro and growth in vivo [Table 3].