Page 299 - Read Online
P. 299
Borniger. J Cancer Metastasis Treat 2019;5:23 I http://dx.doi.org/10.20517/2394-4722.2018.107 Page 3 of 18
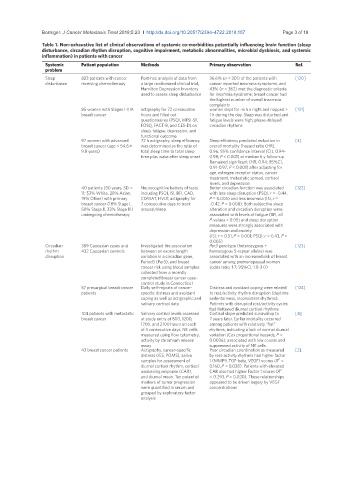
Table 1. Non-exhaustive list of clinical observations of systemic co-morbidities potentially influencing brain function (sleep
disturbance, circadian rhythm disruption, cognitive impairment, metabolic abnormalities, microbial dysbiosis, and systemic
inflammation) in patients with cancer
Systemic Patient population Methods Primary observation Ref.
problem
Sleep 823 patients with cancer Post-hoc analysis of data from 36.6% (n = 301) of the patients with [120]
disturbance receiving chemotherapy a large randomized clinical trial; cancer reported insomnia symptoms, and
Hamilton Depression Inventory 43% (n = 362) met the diagnostic criteria
used to assess sleep disturbance for insomnia syndrome; breast cancer had
the highest number of overall insomnia
complaints
85 women with Stages I-IIIA actigraphy for 72 consecutive women slept for ~6 h a night and napped > [121]
breast cancer hours and filled out 1 h during the day. Sleep was disturbed and
questionnaires (PSQI, MFSI-SF, fatigue levels were high; phase-delayed
FOSQ, FACT-B, and CES-D) on circadian rhythms
sleep, fatigue, depression, and
functional outcome
97 women with advanced 72 h actigraphy; sleep efficiency Sleep efficiency predicted reduction in [3]
breast cancer (age = 54.6 ± was determined as the ratio of overall mortality [hazard ratio (HR),
9.8 years) total sleep time to total sleep 0.96; 95% confidence interval (CI), 0.94-
time plus wake after sleep onset 0.98; P < 0.001] at median 6 y follow-up.
Remained significant (HR, 0.94; 95%CI,
0.91-0.97; P < 0.001) after adjusting for
age, estrogen receptor status, cancer
treatment, metastatic spread, cortisol
levels, and depression
40 patients (50 years, SD = Neurocognitive battery of tests Better circadian function was associated [122]
11; 53% White, 28% Asian, including PSQI, ISI, BFI, CAD, with less sleep disruption (PSQI, r = -0.44,
19% Other) with primary COWAT, HVLT; actigraphy for P = 0.005) and less insomnia (ISI, r =
breast cancer (18% Stage I, 7 consecutive days to track -0.42, P = 0.008). Both subjective sleep
50% Stage II, 33% Stage III) arousal/sleep alteration and circadian disruption were
undergoing chemotherapy associated with levels of fatigue (BFI, all
P-values < 0.05) and sleep disruption
measures were strongly associated with
depression and anxiety
(ISI: r = 0.51, P = 0.001; PSQI: r = 0.43, P =
0.005)
Circadian 389 Caucasian cases and Investigated the association Per3 genotype (heterozygous + [123]
rhythm 432 Caucasian controls between an exonic length homozygous 5-repeat alleles) was
disruption variation in a circadian gene, associated with an increased risk of breast
Period3 (Per3), and breast cancer among premenopausal women
cancer risk using blood samples (odds ratio, 1.7; 95%CI, 1.0-3.0)
collected from a recently
completed breast cancer case-
control study in Connecticut
57 presurgical breast cancer Daily self-reports of cancer- Distress and avoidant coping were related [124]
patients specific distress and avoidant to rest/activity rhythm disruption (daytime
coping as well as actigraphic and sedentariness, inconsistent rhythms).
salivary cortisol data Patients with disrupted rest/activity cycles
had flattened diurnal cortisol rhythms
104 patients with metastatic Salivary cortisol levels assessed Cortisol slope predicted survival up to [4]
breast cancer at study entry at 800, 1200, 7 years later. Earlier mortality occurred
1700, and 2100 hours on each among patients with relatively “flat”
of 3 consecutive days; NK cells rhythms, indicating a lack of normal diurnal
measured using flow cytometry, variation (Cox proportional hazards, P =
activity by chromium release 0.0036); associated with low counts and
assay suppressed activity of NK cells
43 breast cancer patients Actigraphy, cancer-specific Poor circadian coordination as measured [2]
distress (IES, POMS), saliva by rest-activity rhythms had higher factor
2
samples for assessment of 1 (MMP9, TGF-beta, VEGF) scores (R =
diurnal cortisol rhythm, cortisol 0.160, P = 0.038). Patients with elevated
awakening response (CAR), CAR also had higher Factor 1 scores (R 2
and diurnal mean. Ten potential = 0.293, P = 0.020). These relationships
markers of tumor progression appeared to be driven largely by VEGF
were quantified in serum and concentrations
grouped by exploratory factor
analysis