Page 294 - Read Online
P. 294
Page 8 of 10 Bracht et al. J Cancer Metastasis Treat 2019;5:22 I http://dx.doi.org/10.20517/2394-4722.2018.111
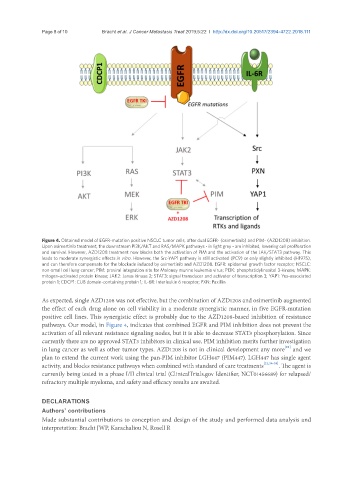
Figure 4. Obtained model of EGFR-mutation positive NSCLC tumor cells, after dual EGFR- (osimertinib) and PIM- (AZD1208) inhibition.
Upon osimertinib treatment, the downstream PI3K/AKT and RAS/MAPK pathways - in light grey - are inhibited, lowering cell proliferation
and survival. However, AZD1208 treatment now blocks both the activation of PIM and the activation of the JAK/STAT3 pathway. This
leads to moderate synergistic effects in vitro. However, the Src-YAP1 pathway is still activated (PC9) or only slightly inhibited (H1975),
and can therefore compensate for the blockade induced by osimertinib and AZD1208. EGFR: epidermal growth factor receptor; NSCLC:
non-small cell lung cancer; PIM: proviral integration site for Moloney murine leukemia virus; PI3K: phosphatidylinositol 3-kinase; MAPK:
mitogen-activated protein kinase; JAK2: Janus kinase 2; STAT3: signal transducer and activator of transcription 3; YAP1: Yes-associated
protein 1; CDCP1: CUB domain-containing protein 1; IL-6R: Interleukin 6 receptor; PXN: Paxillin
As expected, single AZD1208 was not effective, but the combination of AZD1208 and osimertinib augmented
the effect of each drug alone on cell viability in a moderate synergistic manner, in five EGFR-mutation
positive cell lines. This synergistic effect is probably due to the AZD1208-based inhibition of resistance
pathways. Our model, in Figure 4, indicates that combined EGFR and PIM inhibition does not prevent the
activation of all relevant resistance signaling nodes, but it is able to decrease STAT3 phosphorylation. Since
currently there are no approved STAT3 inhibitors in clinical use, PIM inhibition merits further investigation
[22]
in lung cancer as well as other tumor types. AZD1208 is not in clinical development any more and we
plan to extend the current work using the pan-PIM inhibitor LGH447 (PIM447). LGH447 has single agent
activity, and blocks resistance pathways when combined with standard of care treatments [22,36-38] . The agent is
currently being tested in a phase I/II clinical trial (ClinicalTrials.gov Identifier, NCT01456689) for relapsed/
refractory multiple myeloma, and safety and efficacy results are awaited.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and
interpretation: Bracht JWP, Karachaliou N, Rosell R