Page 24 - Read Online
P. 24
CTLA-4 on T-cell responses, [15-18] although there are Table 1: Relation between CTLA-4 gene polymorphisms
contradictory reports on the relationship between +49 A and tumor grade, stage, size, metastasis, estrogen receptor,
to G polymorphisms and cancer development. progesterone receptor and age
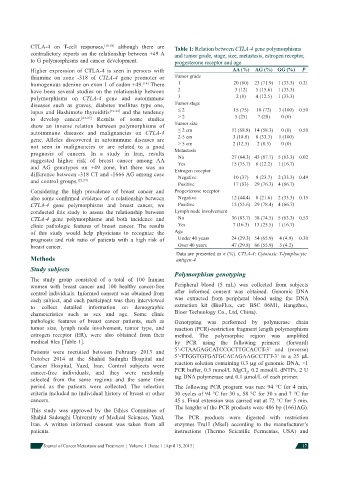
Higher expression of CTLA-4 is seen in persons with AA (%) AG (%) GG (%) P
thiamine on zone -318 of CTLA-4 gene promoter or Tumor grade
homogenous adenine on exon 1 of codon +49. [14] There 1 20 (80) 23 (71.9) 1 (33.3) 0.21
have been several studies on the relationship between 2 3 (12) 5 (15.6) 1 (33.3)
polymorphisms on CTLA-4 gene and autoimmune 3 2 (8) 4 (12.5) 1 (33.3)
diseases such as graves, diabetes mellitus type one, Tumor stage
lupus and Hashimoto thyroiditis [19-22] and the tendency ≤ 2 15 (75) 18 (72) 3 (100) 0.50
to develop cancer. [23-27] Results of some studies > 2 5 (25) 7 (28) 0 (0)
show an inverse relation between polymorphisms of Tumor size
autoimmune diseases and malignancies on CTLA-4 ≤ 2 cm 11 (68.8) 14 (58.3) 0 (0) 0.50
gene. Alleles discovered in autoimmune diseases are 2-5 cm 3 (18.8) 8 (33.3) 1 (100)
not seen in malignancies or are related to a good > 5 cm 2 (12.5) 2 (8.3) 0 (0)
prognosis of cancers. In a study in Iran, results Metastasis 27 (64.3) 43 (87.7) 5 (83.3) 0.02
No
suggested higher risk of breast cancer among AA Yes 15 (35.7) 6 (12.2) 1 (16.7)
and AG genotypes on +49 zone, but there was no Estrogen receptor
difference between -318 CT and -1666 AG among case Negative 10 (37) 9 (23.7) 2 (33.3) 0.49
and control groups. [23,24]
Positive 17 (63) 29 (76.3) 4 (66.7)
Considering the high prevalence of breast cancer and Progesterone receptor
also some confi rmed evidence of a relationship between Negative 12 (44.4) 8 (21.6) 2 (33.3) 0.15
CTLA-4 gene polymorphisms and breast cancer, we Positive 15 (55.6) 29 (78.4) 4 (66.7)
conducted this study to assess the relationship between Lymph node involvement
CTLA-4 gene polymorphisms and both incidence and No 36 (83.7) 38 (74.5) 5 (83.3) 0.53
clinic pathologic features of breast cancer. The results Yes 7 (16.3) 13 (25.5) 1 (16.7)
of this study would help physicians to recognize the Age
prognosis and risk ratio of patients with a high risk of Under 40 years 24 (29.3) 54 (65.9) 4 (4.9) 0.30
breast cancer. Over 40 years 47 (29.8) 66 (55.9) 5 (4.2)
Data are presented as n (%). CTLA-4: Cytotoxic T-lymphocyte
Methods antigen-4
Study subjects
Polymorphism genotyping
The study group consisted of a total of 100 Iranian
women with breast cancer and 100 healthy cancer-free Peripheral blood (5 mL) was collected from subjects
control individuals. Informed consent was obtained from after informed consent was obtained. Genomic DNA
each subject, and each participant was then interviewed was extracted from peripheral blood using the DNA
to collect detailed information on demographic extraction kit (BioFlux, cat: BSC 06M1, Hangzhou,
characteristics such as sex and age. Some clinic Bioer Technology Co., Ltd, China).
pathologic features of breast cancer patients, such as Genotyping was performed by polymerase chain
tumor size, lymph node involvement, tumor type, and reaction (PCR)-restriction fragment length polymorphism
estrogen receptor (ER), were also obtained from their method. The polymorphic region was amplifi ed
medical fi les [Table 1]. by PCR using the following primers: (forward)
5’-CTAAGAGCATCCGCTTGCACCT-3’ and (reverse)
Patients were recruited between February 2013 and
October 2014 at the Shahid Sadughi Hospital and 5’-TTGGTGTGATGCACAGAAGCCTTT-3’ in a 25 L
Cancer Hospital, Yazd, Iran. Control subjects were reaction solution containing 0.3 g of genomic DNA, ×1
cancer-free individuals, and they were randomly PCR buffer, 0.3 mmol/L MgCl , 0.2 mmol/L dNTPs, 2 U
2
selected from the same regions and the same time tag DNA polymerase and 0.1 mol/L of each primer.
period as the patients were collected. The selection The following PCR program was run: 94 °C for 4 min,
criteria included no individual history of breast or other 30 cycles of 94 °C for 30 s, 58 °C for 30 s and 7 °C for
cancers. 45 s. Final extension was carried out at 72 °C for 5 min.
The lengths of the PCR products were 486 bp (1661AG).
This study was approved by the Ethics Committee of
Shahid Sadoughi University of Medical Sciences, Yazd, The PCR products were digested with restriction
Iran. A written informed consent was taken from all enzymes Tru1I (MseI) according to the manufacturer’s
patients. instructions (Thermo Scientifi c Fermentas, USA) and
Journal of Cancer Metastasis and Treatment ¦ Volume 1 ¦ Issue 1 ¦ April 15, 2015 ¦ 17