Page 90 - Read Online
P. 90
Skorupan et al. J Cancer Metastasis Treat 2023;9:5 https://dx.doi.org/10.20517/2394-4722.2022.106 Page 19 of 26
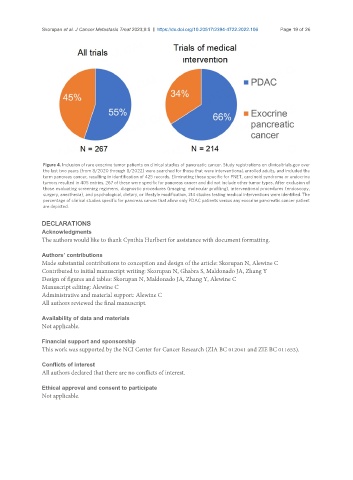
Figure 4. Inclusion of rare exocrine tumor patients on clinical studies of pancreatic cancer. Study registrations on clinicaltrials.gov over
the last two years (from 8/2020 through 8/2022) were searched for those that were interventional, enrolled adults, and included the
term pancreas cancer, resulting in identification of 425 records. Eliminating those specific for PNET, carcinoid syndrome or endocrine
tumors resulted in 405 entries. 267 of these were specific for pancreas cancer and did not include other tumor types. After exclusion of
those evaluating screening regimens, diagnostic procedures (imaging, molecular profiling), interventional procedures (endoscopy,
surgery, anesthesia), and psychological, dietary, or lifestyle modification, 214 studies testing medical interventions were identified. The
percentage of clinical studies specific for pancreas cancer that allow only PDAC patients versus any exocrine pancreatic cancer patient
are depicted.
DECLARATIONS
Acknowledgments
The authors would like to thank Cynthia Hurlbert for assistance with document formatting.
Authors’ contributions
Made substantial contributions to conception and design of the article: Skorupan N, Alewine C
Contributed to initial manuscript writing: Skorupan N, Ghabra S, Maldonado JA, Zhang Y
Design of figures and tables: Skorupan N, Maldonado JA, Zhang Y, Alewine C
Manuscript editing: Alewine C
Administrative and material support: Alewine C
All authors reviewed the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the NCI Center for Cancer Research (ZIA BC 012041 and ZIE BC 011653).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.