Page 83 - Read Online
P. 83
Page 12 of 26 Skorupan et al. J Cancer Metastasis Treat 2023;9:5 https://dx.doi.org/10.20517/2394-4722.2022.106
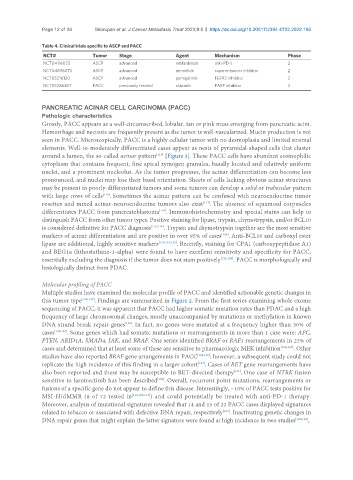
Table 4. Clinical trials specific to ASCP and PACC
NCT# Tumor Stage Agent Mechanism Phase
NCT04116073 ASCP advanced retifanlimab anti-PD-1 2
NCT04896073 ASCP advanced minnelide superenhancer inhibitor 2
NCT05216120 ASCP advanced pemigatinib FGFR2 inhibitor 2
NCT05286827 PACC previously treated olaparib PARP inhibitor 2
PANCREATIC ACINAR CELL CARCINOMA (PACC)
Pathologic characteristics
Grossly, PACC appears as a well-circumscribed, lobular, tan or pink mass emerging from pancreatic acini.
Hemorrhage and necrosis are frequently present as the tumor is well-vascularized. Mucin production is not
seen in PACC. Microscopically, PACC is a highly cellular tumor with no desmoplasia and limited stromal
elements. Well-to-moderately differentiated cases appear as nests of pyramidal-shaped cells that cluster
[115]
around a lumen, the so-called acinar pattern [Figure 3]. These PACC cells have abundant eosinophilic
cytoplasm that contains frequent, fine apical zymogen granules, basally located and relatively uniform
nuclei, and a prominent nucleolus. As the tumor progresses, the acinar differentiation can become less
pronounced, and nuclei may lose their basal orientation. Sheets of cells lacking obvious acinar structures
may be present in poorly-differentiated tumors and some tumors can develop a solid or trabecular pattern
[116]
with large rows of cells . Sometimes the acinar pattern can be confused with neuroendocrine tumor
rosettes and mixed acinar-neuroendocrine tumors also exist . The absence of squamoid corpuscles
[117]
[118]
differentiates PACC from pancreatoblastoma . Immunohistochemistry and special stains can help to
distinguish PACC from other tumor types. Positive staining for lipase, trypsin, chymotrypsin, and/or BCL10
is considered definitive for PACC diagnosis [115,119] . Trypsin and chymotrypsin together are the most sensitive
markers of acinar differentiation and are positive in over 95% of cases . Anti-BCL10 and carboxyl ester
[120]
lipase are additional, highly sensitive markers [119,121,122] . Recently, staining for CPA1 (carboxypeptidase A1)
and REG1α (lithostathine-1-alpha) were found to have excellent sensitivity and specificity for PACC,
essentially excluding the diagnosis if the tumor does not stain positively [123,124] . PACC is morphologically and
histologically distinct from PDAC.
Molecular profiling of PACC
Multiple studies have examined the molecular profile of PACC and identified actionable genetic changes in
this tumor type [125-127] . Findings are summarized in Figure 2. From the first series examining whole exome
sequencing of PACC, it was apparent that PACC had higher somatic mutation rates than PDAC and a high
frequency of large chromosomal changes, mostly unaccompanied by mutations or methylation in known
[125]
DNA strand break repair genes . In fact, no genes were mutated at a frequency higher than 30% of
cases [125,127] . Some genes which had somatic mutations or rearrangements in more than 1 case were: APC,
PTEN, ARID1A, SMAD4, JAK, and BRAF. One series identified BRAF or RAF1 rearrangements in 23% of
cases and determined that at least some of these are sensitive to pharmacologic MEK inhibition [126,128] . Other
studies have also reported BRAF gene arrangements in PACC [129,130] ; however, a subsequent study could not
[127]
replicate the high incidence of this finding in a larger cohort . Cases of RET gene rearrangements have
also been reported and these may be susceptible to RET-directed therapy . One case of NTRK fusion
[131]
[132]
sensitive to larotrectinib has been described . Overall, recurrent point mutations, rearrangements or
fusions of a specific gene do not appear to define this disease. Interestingly, ~10% of PACC tests positive for
MSI-H/dMMR (8 of 72 tested in [118,125,133] ) and could potentially be treated with anti-PD-1 therapy.
Moreover, analysis of mutational signatures revealed that 14 and 15 of 22 PACC cases displayed signatures
related to tobacco or associated with defective DNA repair, respectively . Inactivating genetic changes in
[127]
DNA repair genes that might explain the latter signature were found at high incidence in two studies [126,128] ,