Page 71 - Read Online
P. 71
Page 4 of 12 Yonemura et al. J Cancer Metastasis Treat 2022;8:43 https://dx.doi.org/10.20517/2394-4722.2022.49
Table 1. Treatment results of systemic chemotherapy for DMPM
Authors, No of Response 1-year Median Side effects
years Study type Eligibility case Regimen rates survial survival grade 3,4
published (months)
Cartenni et al., Nonrandomized DMPM 37 PEM + CDDP 0 1 NA Neutropenia; 60%
[13]
2009
Open-label study Not amenable to 34 PEM + 0 NR NA Anemia 5%
CBDCA
Curative surgery 38 PEM 0 0 13 Fatigue 10%,
dehydration; 10%
Janne et al., Nonrandomized DMPM 32 PEM + CDDP 0 1 13 Bone marrow;
[14]
2005 0.1%-2.5%
Open-label study Not amenable to 66 PEM 0 0 9 Dehydration 7.25
Curative surgery Digestive tract:
3.8%-7.2%
Simon et al., Phase II Not amenable to 20 GEM + PEM 0 1 27 Neutropenia; 60%
2009 [15]
Curative surgery One patient;
Grade 5
Le et al., 2003 [16] Phase III DMPM 62 Irrinitecan + 0 1 NR No Grade 3,4 side
CDDP effect
Sgarbura et al., Phase II PCI > 27, small 44 PIPAC + SC UI UI UI UI
[17]
2009 bowel PCI > 4
22 SC
Fennell et al., Phase III Mesothelioma 221 Nivolumab 0 0 10 1%-3% (Grade 3)
[18]
2021 randomized
Pleural 318, non 113 Placebo 0 0 7 0%-2% (Grade3)
pleural 16
DMPM: Diffuse malignant peritoneal mesothelioma; UI: under investigation; SC: systemic chemotherapy.
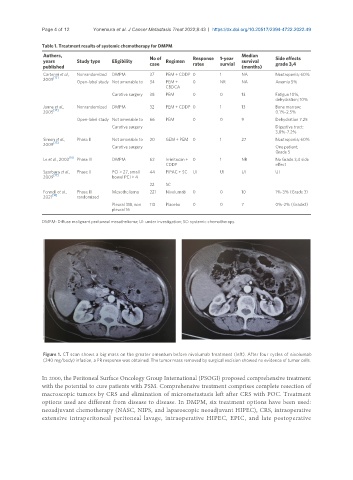
Figure 1. CT scan shows a big mass on the greater omentum before nivolumab treatment (left). After four cycles of nivolumab
(240 mg/body) infusion, a PR response was obtained. The tumor mass removed by surgical excision showed no evidence of tumor cells.
In 2000, the Peritoneal Surface Oncology Group International (PSOGI) proposed comprehensive treatment
with the potential to cure patients with PSM. Comprehensive treatment comprises complete resection of
macroscopic tumors by CRS and elimination of micrometastasis left after CRS with POC. Treatment
options used are different from disease to disease. In DMPM, six treatment options have been used:
neoadjuvant chemotherapy (NASC, NIPS, and laparoscopic neoadjuvant HIPEC), CRS, intraoperative
extensive intraperitoneal peritoneal lavage, intraoperative HIPEC, EPIC, and late postoperative