Page 109 - Read Online
P. 109
Offin et al. J Cancer Metastasis Treat 2023;9:21 https://dx.doi.org/10.20517/2394-4722.2022.140 Page 7 of 16
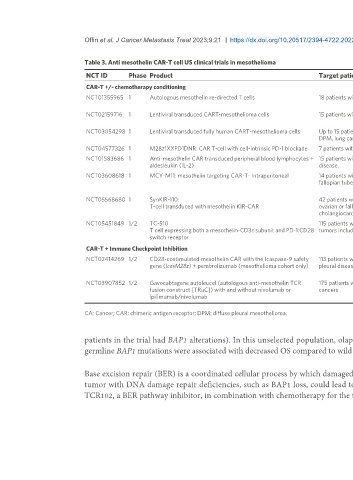
Table 3. Anti mesothelin CAR-T cell US clinical trials in mesothelioma
NCT ID Phase Product Target patient population Outcomes Reference
CAR-T +/- chemotherapy conditioning
NCT01355965 1 Autologous mesothelin re-directed T cells 18 patients with DPM. 4 Patients treated with anaphylaxis, off-target [70,71]
toxicity
NCT02159716 1 Lentiviral transduced CART-mesothelioma cells 15 patients with DPM, ovarian ca, pancreatic ductal ca. Cells well tolerated, expanded in blood, limited [72]
clinical activity
NCT03054298 1 Lentiviral transduced fully human CART-mesothelioma cells Up to 15 patients with mesothelin-expressing refractory Study Ongoing
DPM, lung cancer, and ovarian ca.
NCT04577326 1 M28z1XXPD1DNR: CAR T-cell with cell-intrinsic PD-1 blockade 7 patients with DPM. Study Ongoing [73]
NCT01583686 1 Anti-mesothelin CAR transduced peripheral blood lymphocytes + 15 patients with mesothelin expressing metastatic Study Terminated for poor accrual
aldesleukin (IL-2) disease.
NCT03608618 1 MCY-M11: mesothelin targeting CAR-T- Intraperitoneal 14 patients with ovarian Ca, primary peritoneal or Following the treatment of 11 patients with initial [74]
fallopian tube ca, and peritoneal mesothelioma. feasibility and safety reported, study terminated-
sponsor priority.
NCT05568680 1 SynKIR-110: 42 patients with ovarian Ca, primary peritoneal Ca, Study Ongoing
T-cell transduced with mesothelin KIR-CAR ovarian or fallopian tube Ca, mesotheliomas,
cholangiocarcinoma
NCT05451849 1/2 TC-510 115 patients with advanced mesothelin-expressing Study Ongoing
T cell expressing both a mesothelin-CD3ε subunit and PD-1:CD28 tumors including DPM
switch receptor
CAR-T + Immune Checkpoint Inhibition
NCT02414269 1/2 CD28-costimulated mesothelin CAR with the Icaspase-9 safety 113 patients with mesothelin expressing malignant 19 DPM patients: 2 complete metabolic response [75]
gene (IcasM28z) + pembrolizumab (mesothelioma cohort only) pleural disease. on PET, 5 partial response, 4 stable disease.
Study Ongoing.
NCT03907852 1/2 Gavocabtagene autoleucel (autologous anti-mesothelin TCR 175 patients with advanced mesothelin-expressing Tumor regression in first 5 patients treated. [76]
fusion construct [TRuC]) with and without nivolumab or cancers
ipilimumab/nivolumab Study ongoing.
CA: Cancer; CAR: chimeric antigen receptor; DPM: diffuse pleural mesothelioma.
patients in the trial had BAP1 alterations). In this unselected population, olaparib had limited activity, with one (4%) partial response. In this small sample,
germline BAP1 mutations were associated with decreased OS compared to wild type (4.6 vs. 9.6 months, respectively, P = 0.004).
Base excision repair (BER) is a coordinated cellular process by which damaged DNA base pairs can be excised and repaired ; inhibition of this pathway in a
[94]
tumor with DNA damage repair deficiencies, such as BAP1 loss, could lead to synthetic lethality. A recent phase 1 trial examined the safety and activity of
TCR102, a BER pathway inhibitor, in combination with chemotherapy for the treatment of multiple advanced solid tumors . In the DPM cohort, 14 patients
[87]