Page 105 - Read Online
P. 105
Offin et al. J Cancer Metastasis Treat 2023;9:21 https://dx.doi.org/10.20517/2394-4722.2022.140 Page 3 of 16
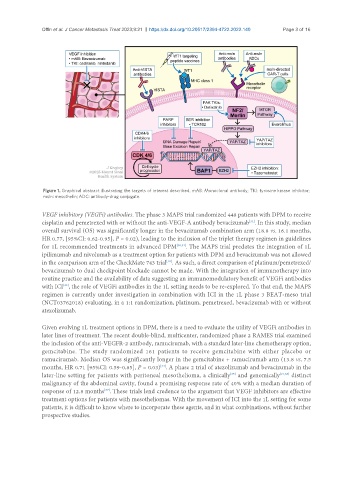
Figure 1. Graphical abstract illustrating the targets of interest described. mAB: Monoclonal antibody; TKI: tyrosine kinase inhibitor;
msln: mesothelin; ADC: antibody-drug conjugate.
VEGF inhibitory (VEGFi) antibodies. The phase 3 MAPS trial randomized 448 patients with DPM to receive
cisplatin and pemetrexed with or without the anti-VEGF-A antibody bevacizumab . In this study, median
[31]
overall survival (OS) was significantly longer in the bevacizumab combination arm (18.8 vs. 16.1 months,
HR 0.77, [95%CI: 0.62-0.95], P = 0.02), leading to the inclusion of the triplet therapy regimen in guidelines
for 1L recommended treatments in advanced DPM [30,32] . The MAPS trial predates the integration of 1L
ipilimumab and nivolumab as a treatment option for patients with DPM and bevacizumab was not allowed
in the comparison arm of the CheckMate 743 trial . As such, a direct comparison of platinum/pemetrexed/
[18]
bevacizumab to dual checkpoint blockade cannot be made. With the integration of immunotherapy into
routine practice and the availability of data suggesting an immunomodulatory benefit of VEGFi antibodies
[33]
with ICI , the role of VEGFi antibodies in the 1L setting needs to be re-explored. To that end, the MAPS
regimen is currently under investigation in combination with ICI in the 1L phase 3 BEAT-meso trial
(NCT03762018) evaluating, in a 1:1 randomization, platinum, pemetrexed, bevacizumab with or without
atezolizumab.
Given evolving 1L treatment options in DPM, there is a need to evaluate the utility of VEGFi antibodies in
later lines of treatment. The recent double-blind, multicenter, randomized phase 2 RAMES trial examined
the inclusion of the anti-VEGFR-2 antibody, ramucirumab, with a standard later-line chemotherapy option,
gemcitabine. The study randomized 161 patients to receive gemcitabine with either placebo or
ramucirumab. Median OS was significantly longer in the gemcitabine + ramucirumab arm (13.8 vs. 7.5
months, HR 0.71 [95%CI: 0.59-0.85], P = 0.03) . A phase 2 trial of atezolizumab and bevacizumab in the
[34]
[35]
later-line setting for patients with peritoneal mesothelioma, a clinically and genomically [21,36] distinct
malignancy of the abdominal cavity, found a promising response rate of 40% with a median duration of
response of 12.8 months . These trials lend credence to the argument that VEGF inhibitors are effective
[37]
treatment options for patients with mesotheliomas. With the movement of ICI into the 1L setting for some
patients, it is difficult to know where to incorporate these agents, and in what combinations, without further
prospective studies.