Page 111 - Read Online
P. 111
Page 8 of 16 Offin et al. J Cancer Metastasis Treat 2023;9:21 https://dx.doi.org/10.20517/2394-4722.2022.140
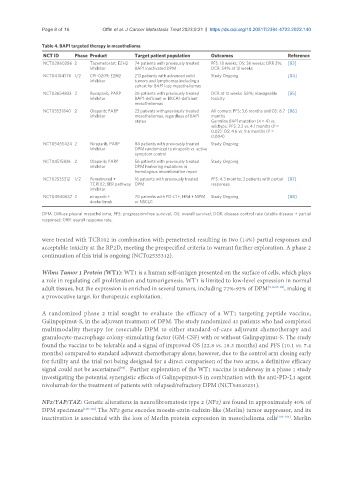
Table 4. BAP1 targeted therapy in mesothelioma
NCT ID Phase Product Target patient population Outcomes Reference
NCT02860286 2 Tazemetostat; EZH2 74 patients with previously treated PFS: 18 weeks; OS: 36 weeks; ORR 3%; [83]
inhibitor BAP1 inactivated DPM DCR: 54% at 12 weeks
NCT04104776 1/2 CPI-0209; EZH2 213 patients with advanced solid Study Ongoing [84]
inhibitor tumors and lymphomas including a
cohort for BAP1 loss mesotheliomas
NCT03654833 2 Rucaparib; PARP 26 patients with previously treated DCR at 12 weeks: 58%; manageable [85]
inhibitor BAP1-deficient or BRCA1-deficient toxicity
mesotheliomas
NCT03531840 2 Olaparib; PARP 23 patients with previously treated All comers: PFS: 3.6 months and OS: 8.7 [86]
inhibitor mesotheliomas, regardless of BAP1 months
status Germline BAP1 mutation (n = 4) vs.
wildtype: PFS: 2.3 vs. 4.1 months (P =
0.02); OS: 4.6 vs. 9.6 months (P =
0.004)
NCT05455424 2 Niraparib; PARP 84 patients with previously treated Study Ongoing
inhibitor DPM randomized to niraparib vs. active
symptom control
NCT04515836 2 Olaparib; PARP 56 patients with previously treated Study Ongoing
inhibitor DPM harboring mutations in
homologous recombination repair
NCT02535312 1/2 Pemetrexed + 16 patients with previously treated PFS: 4.3 months; 2 patients with partial [87]
TCR102; BER pathway DPM responses
inhibitor
NCT04940637 2 niraparib + 70 patients with PD-L1 +, HRd + MPM Study Ongoing [88]
dostarlimab or NSCLC
DPM: Diffuse pleural mesothelioma; PFS: progression-free survival; OS: overall survival; DCR: disease control rate (stable disease + partial
response); ORR: overall response rate.
were treated with TCR102 in combination with pemetrexed resulting in two (14%) partial responses and
acceptable toxicity at the RP2D, meeting the prespecified criteria to warrant further exploration. A phase 2
continuation of this trial is ongoing (NCT02535312).
Wilms Tumor 1 Protein (WT1): WT1 is a human self-antigen presented on the surface of cells, which plays
a role in regulating cell proliferation and tumorigenesis. WT1 is limited to low-level expression in normal
adult tissues, but the expression is enriched in several tumors, including 72%-93% of DPM [9,10,95-98] , making it
a provocative target for therapeutic exploitation.
A randomized phase 2 trial sought to evaluate the efficacy of a WT1 targeting peptide vaccine,
Galinpepimut-S, in the adjuvant treatment of DPM. The study randomized 41 patients who had completed
multimodality therapy for resectable DPM to either standard-of-care adjuvant chemotherapy and
granulocyte-macrophage colony-stimulating factor (GM-CSF) with or without Galinpepimut-S. The study
found the vaccine to be tolerable and a signal of improved OS (22.8 vs. 18.3 months) and PFS (10.1 vs. 7.4
months) compared to standard adjuvant chemotherapy alone; however, due to the control arm closing early
for futility and the trial not being designed for a direct comparison of the two arms, a definitive efficacy
signal could not be ascertained . Further exploration of the WT1 vaccine is underway in a phase 1 study
[99]
investigating the potential synergistic effects of Galinpepimut-S in combination with the anti-PD-L1 agent
nivolumab for the treatment of patients with relapsed/refractory DPM (NCT04040231).
NF2/YAP/TAZ: Genetic alterations in neurofibromatosis type 2 (NF2) are found in approximately 40% of
DPM specimens [100-102] .The NF2 gene encodes moesin-ezrin-radixin-like (Merlin) tumor suppressor, and its
inactivation is associated with the loss of Merlin protein expression in mesothelioma cells [103-105] . Merlin