Page 113 - Read Online
P. 113
Page 10 of 16 Offin et al. J Cancer Metastasis Treat 2023;9:21 https://dx.doi.org/10.20517/2394-4722.2022.140
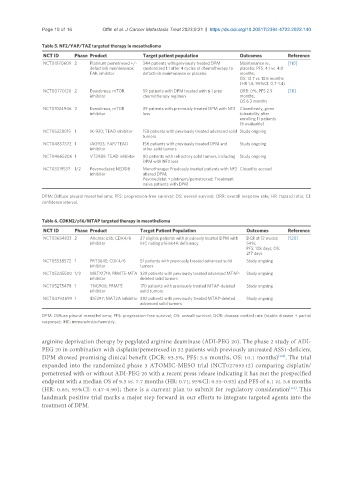
Table 5. NF2/YAP/TAZ targeted therapy in mesothelioma
NCT ID Phase Product Target patient population Outcomes Reference
NCT01870609 2 Platinum pemetrexed +/- 344 patients with previously treated DPM Maintenance vs. [110]
defactinib maintenance; randomized 1:1 after 4 cycles of chemotherapy to placebo: PFS: 4.1 vs. 4.0
FAK inhibitor defactinib maintenance or placebo months;
OS: 12.7 vs. 13.6 months
(HR 1.0, 95%CI: 0.7-1.4)
NCT00770120 2 Everolimus; mTOR 59 patients with DPM treated with ≤ 1 prior ORR: 0%; PFS 2.9 [111]
inhibitor chemotherapy regimen months;
OS 6.3 months
NCT01024946 2 Everolimus; mTOR 39 patients with previously treated DPM with NF2 Closed early, given
inhibitor loss tolerability after
enrolling 11 patients
(6 evaluable)
NCT05228015 1 IK-930; TEAD inhibitor 158 patients with previously treated advanced solid Study ongoing
tumors
NCT04857372 1 IAG933; YAP/TEAD 156 patients with previously treated DPM and Study ongoing
inhibitor other solid tumors
NCT04665206 1 VT3989; TEAD inhibitor 80 patients with refractory solid tumors, including Study ongoing
DPM with NF2 loss
NCT03319537 1/2 Pevonedistat; NEDD8 Monotherapy: Previously treated patients with NF2 Closed to accrual
inhibitor altered DPM;
Pevonedistat + platinum/pemetrexed: Treatment
naïve patients with DPM
DPM: Diffuse pleural mesothelioma; PFS: progression-free survival; OS: overall survival; ORR: overall response rate; HR: hazard ratio; CI:
confidence interval.
Table 6. CDKN2/p16/MTAP targeted therapy in mesothelioma
NCT ID Phase Product Target Patient Population Outcomes Reference
NCT03654833 2 Abemaciclib; CDK4/6 27 eligible patients with previously treated DPM with DCR at 12 weeks: [120]
inhibitor IHC noting p16ink4A deficiency 54%;
PFS: 128 days; OS:
217 days
NCT05538572 1 PRT3645; CDK4/6 51 patients with previously treated advanced solid Study ongoing
inhibitor tumors
NCT05245500 1/2 MRTX1719; PRMT5-MTA 339 patients with previously treated advanced MTAP- Study ongoing
inhibitor deleted solid tumors
NCT05275478 1 TNG908; PRMT5 170 patients with previously treated MTAP-deleted Study ongoing
inhibitor solid tumors
NCT04794699 1 IDE397; MAT2A Inhibitor 382 patients with previously treated MTAP-deleted Study ongoing
advanced solid tumors
DPM: Diffuse pleural mesothelioma; PFS: progression-free survival; OS: overall survival; DCR: disease control rate (stable disease + partial
response); IHC: immunohistochemistry.
arginine deprivation therapy by pegylated arginine deaminase (ADI-PEG 20). The phase 2 study of ADI-
PEG 20 in combination with cisplatin/pemetrexed in 32 patients with previously untreated ASS1-deficient
DPM showed promising clinical benefit (DCR: 93.5%, PFS: 5.6 months, OS: 10.1 months) . The trial
[132]
expanded into the randomized phase 3 ATOMIC-MESO trial (NCT02709512) comparing cisplatin/
pemetrexed with or without ADI-PEG 20 with a recent press release indicating it has met the prespecified
endpoint with a median OS of 9.3 vs. 7.7 months (HR: 0.71; 95%CI: 0.55-0.93) and PFS of 6.1 vs. 5.6 months
[133]
(HR: 0.65; 95%CI: 0.47-0.90); there is a current plan to submit for regulatory consideration . This
landmark positive trial marks a major step forward in our efforts to integrate targeted agents into the
treatment of DPM.