Page 31 - Read Online
P. 31
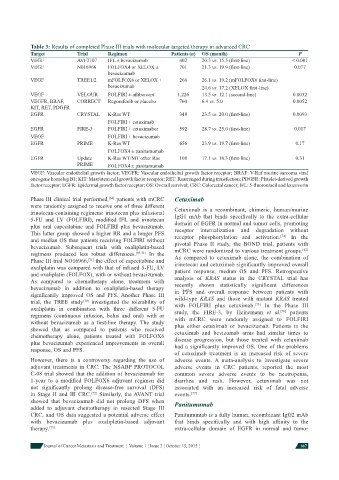
Table 3: Results of completed Phase III trials with molecular targeted therapy in advanced CRC
Target Trial Regimen Patients (n) OS (month) P
VEGF AVF2107 IFL ± bevacizumab 402 20.3 vs. 15.5 (fi rst-line) < 0.001
VEGF N016966 FOLFOX4 or XELOX ± 701 21.3 vs. 19.9 (fi rst-line) 0.077
bevacizumab
VEGF TREE1/2 mFOLFOX6 or XELOX ± 260 26.1 vs. 19.2 (mFOLFOX6 fi rst-line)
bevacizumab 24.6 vs. 17.2 (XELOX fi rst-line)
VEGF VELOUR FOLFIRI ± afl ibercept 1,226 13.5 vs. 12.1 (second-line) 0.0032
VEGFR, BRAF, CORRECT Regorafenib or placebo 760 6.4 vs. 5.0 0.0052
KIT, RET, PDGFR
EGFR CRYSTAL K-Ras WT 348 23.5 vs. 20.0 (fi rst-line) 0.0093
FOLFIRI ± cetuximab
EGFR FIRE-3 FOLFIRI ± cetuximabor 592 28.7 vs. 25.0 (fi rst-line) 0.017
VEGF FOLFIRI ± bevacizumab
EGFR PRIME K-Ras WT 656 23.9 vs. 19.7 (fi rst-line) 0.17
FOLFOX4 ± panitumumab
EGFR Update K-Ras WT/MT other Ras 108 17.1 vs. 18.3 (fi rst-line) 0.31
PRIME FOLFOX4 ± panitumumab
VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; BRAF: V-Raf murine sarcoma viral
oncogene homolog B1; KIT: Mast/stem cell growth factor receptor; RET: Rearranged during transfection; PDGFR: Platelet-derived growth
factor receptor; EGFR: Epidermal growth factor receptor; OS: Overall survival; CRC: Colorectal cancer; IFL: 5-fl uorouracil and leucovorin
[68]
Phase III clinical trial performed, patients with mCRC Cetuximab
were randomly assigned to receive one of three different Cetuximab is a recombinant, chimeric, human/murine
irinotecan-containing regimens: irinotecan plus infusional IgG1 mAb that binds specifi cally to the extra-cellular
5-FU and LV (FOLFIRI), modifi ed IFL and irinotecan domain of EGFR in normal and tumor cells, promoting
plus oral capecitabine and FOLFIRI plus bevacizumab. receptor internalization and degradation without
This latter group showed a higher RR and a longer PFS receptor phosphorylation and activation. [74] In the
and median OS than patients receiving FOLFIRI without pivotal Phase II study, the BOND trial, patients with
bevacizumab. Subsequent trials with oxaliplatin-based mCRC were randomized to various treatment groups. [62]
regimens produced less robust differences. [69-71] In the As compared to cetuximab alone, the combination of
Phase III trial NO16966, the effect of capecitabine and irinotecan and cetuximab signifi cantly improved overall
[71]
oxaliplatin was compared with that of infused 5-FU, LV patient response, median OS and PFS. Retrospective
and oxaliplatin (FOLFOX), with or without bevacizumab. analysis of KRAS status in the CRYSTAL trial has
As compared to chemotherapy alone, treatment with recently shown statistically signifi cant differences
bevacizumab in addition to oxaliplatin-based therapy in PFS and overall response between patients with
signifi cantly improved OS and PFS. Another Phase III wild-type KRAS and those with mutant KRAS treated
trial, the TREE study investigated the tolerability of with FOLFIRI plus cetuximab. [75] In the Phase III
[70]
oxaliplatin in combination with three different 5-FU study, the FIRE-3, by Heinemann et al. patients
[76]
regimens (continuous infusion, bolus and oral) with or with mCRC were randomly assigned to FOLFIRI
without bevacizumab as a fi rst-line therapy. The study plus either cetuximab or bevacizumab. Patients in the
showed that as compared to patients who received cetuximab and bevizumab arms had similar times to
chemotherapy alone, patients treated with FOLFOX6 disease progression, but those treated with cetuximab
plus bevacizumab experienced improvements in overall had a signifi cantly improved OS. One of the problems
response, OS and PFS.
of cetuximab treatment is an increased risk of severe
However, there is a controversy regarding the use of adverse events. A meta-analysis to investigate severe
adjuvant treatments in CRC. The NSABP PROTOCOL adverse events in CRC patients, reported the most
C-08 trial showed that the addition of bevacizumab for common severe adverse events to be neutropenia,
1-year to a modifi ed FOLFOX6 adjuvant regimen did diarrhea and rash. However, cetuximab was not
not signifi cantly prolong disease-free survival (DFS) associated with an increased risk of fatal adverse
in Stage II and III CRC. [72] Similarly, the AVANT trial events. [77]
showed that bevacizumab did not prolong DFS when Panitumumab
added to adjuvant chemotherapy in resected Stage III
CRC, and OS data suggested a potential adverse effect Panitumumab is a fully human, recombinant IgG2 mAb
with bevacizumab plus oxaliplatin-based adjuvant that binds specifi cally and with high affi nity to the
therapy. [73] extra-cellular domain of EGFR in normal and tumor
Journal of Cancer Metastasis and Treatment ¦ Volume 1 ¦ Issue 3 ¦ October 15, 2015 ¦ 167