Page 77 - Read Online
P. 77
Page 12 of 19 Serzan et al. J Cancer Metastasis Treat 2021;7:39 https://dx.doi.org/10.20517/2394-4722.2021.76
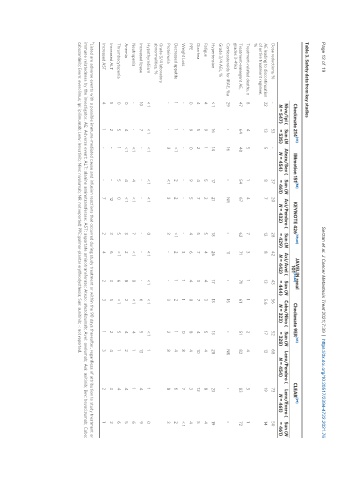
Table 3. Safety data from key studies
Checkmate 214 [43] IMmotion 151 [58] KEYNOTE 426 [59,60] JAVELIN renal Checkmate 9ER [63] CLEAR [29]
[61,62]
101
Nivo/Ipi ( Sun (N Atezo/Bev ( Sun (N Axi/Pembro ( Sun (N Axi/Avel ( Sun (N Cabo/Nivo ( Sun (N Lenv/Pembro ( Lenv/Evero ( Sun (N
N = 547) = 535) N = 454) = 461) N = 432) = 429) N = 442) = 444) N = 323) = 328) N = 454) N = 461) = 461)
Dose reductions, % - 53 - 37 20 28 42 43 56 52 68 73 50
AE leading to discontinuation 22 13 5 8 7 12 8 13 5.6 17 13 19 14
of entire treatment regimen,
%
Treatment-related deaths, n 8 4 5 1 4 7 3 1 1 2 4 3 1
Treatment-emergent AE, 47 64 40 54 67 62 71 71 61 51 82 83 72
grades 3-4%a
Corticosteroids for IRAE, %a 29 - 16 - NR - 11 - 16 - NR - -
Grade 3/4 AEs, %
Hypertension < 1 16 14 17 21 18 26 17 13 13 28 23 19
Fatigue 4 9 1 5 2 5 4 4 3 5 4 8 4
Diarrhea 4 5 2 4 7 5 7 3 7 4 10 12 5
PPE 0 9 0 9 5 4 6 4 8 8 4 3 4
Weight Loss - - - - - - 3 1 1 0 8 7 < 1
Decreased appetite 1 1 < 1 2 2 < 1 2 1 2 1 4 6 2
Proteinuria - - 3 < 1 3 3 - - 3 2 8 8 3
Grade 3/4 laboratory
abnormalities, %
Hypothyrodiism < 1 < 1 < 1 < 1 < 1 0 < 1 < 1 < 1 < 1 1 1 0
Increased lipase 10 7 - - - - - - 6 5 13 4 9
Neutropenia - - < 1 4 < 1 7 < 1 8 < 1 4 1 1 6
Anemia 0 4 < 1 4 < 1 3 2 8 2 4 2 4 5
Thrombocytopenia 0 5 1 5 0 5 < 1 6 < 1 5 1 4 6
Increased ALT 5 2 - - 12 3 6 3 5 2 4 3 2
Increased AST 4 1 - - 7 2 4 2 3 1 3 2 1
a
Listed are adverse events with a possible immune-mediated cause and infusion reactions that occurred during study treatment or within the 90 days thereafter, regardless of attribution to study treatment or
immune relatedness by the investigator. AE: Adverse event; ALT: alanine aminotransferase; AST: aspartate aminotransferase; Atezo: atezolizumab; Avel: avelumab; Axi: axitinib; Bev: bevacizumab; Cabo:
cabozantinib; Evero: everolimus; Ipi: ipilimumab; Lenv: lenvatinib; Nivo: nivolumab; NR: not reported; PPE: palmar-plantar erythrodysthesia; Sun: sunitinib; -: not reported.