Page 80 - Read Online
P. 80
Serzan et al. J Cancer Metastasis Treat 2021;7:39 https://dx.doi.org/10.20517/2394-4722.2021.76 Page 15 of 19
Table 4. Current clincial trails for first line ccRCC
Study Key results/completion
Title Patients Regimen Study details
design status
Systemic therapy
Checkmate 8Y8 1L, Phase Int/poor risk (N Nivo Co-primary endpoint: PFS Estimated study completion:
[48]
(NCT03873402) 3 = 418) Nivo/Ipi and ORR by BICR date April 2025
COSMIC 313 1L, Phase Int/poor risk (N Cabo/Nivo Primary endpoint: duration Estimated study completion
[81]
(NCT03937219) 3 = 840) Cabo/Nivo/Ipi PFS by BICR date: March 2025
PD1GREE 1L, Phase Int/poor risk (N Nivo/Ipi induction, Primary endpoint: OS Estimated study completion
[82]
(NCT03793166) 3 = 1046) Nivo date: April 2022
Nivo/Cabo
Surgery/radiation
NORDIC-SUN 1L, Phase Int/poor risk (N Nivo 3mg/kg/Ipi Primary endpoint: OS Estimated study completion
[77]
(NCT03977571) 3 = 400) 1mg/kg q3wk x4 date: September 2025
- Delayed CRN/Nivo
- Nivo
CYTOSHRINK 1L, Phase Int/poor risk (N Nivo 3 mg/kg/Ipi 1 Primary endpoint: PFS Estimated study completion
[78]
(NCT04090710) 2 = 78) mg/kg q3wk x4 date: April 2022
- SBRT prior to cycle 2
- no SBRT
Cyto-KIK trial 1L, Phase Treatment naive Nivo 480 mg Primary endpoint: % of Estimated study completion
(NCT04322955) [79] 2 (N = 48) q4wk/Cabo 40 mg qd participants with a complete date: February 2022
- Delayed CRN response
PROBE trial 1L, Phase Treatment naive Initial CRN then Primary endpoint: OS Estimated study completion
[80]
(NCT04510597) 3 (N = 364) systemic therapy date: July 2033
Systemic therapy
alone
Cabo: Cabozantinib; CRN: cytoreductive nephrectomy; IMDC: International Metastatic Renal Cell Carcinoma; int: intermediate; Ipi: ipilimumab;
Nivo: nivolumab; ORR: objective response rate; OS: overall survival; PFS: progression free survival; Ph: phase; q3wk: every 3 weeks; qd: once daily;
wk: week; SBRT: stereotactic body radiation therapy.
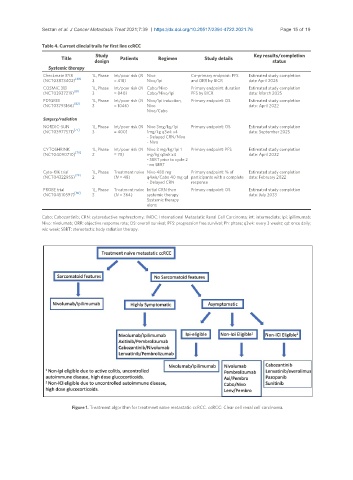
Figure 1. Treatment algorithm for treatmnet naïve metastatic ccRCC. ccRCC: Clear cell renal cell carcinoma.