Page 72 - Read Online
P. 72
Serzan et al. J Cancer Metastasis Treat 2021;7:39 https://dx.doi.org/10.20517/2394-4722.2021.76 Page 7 of 19
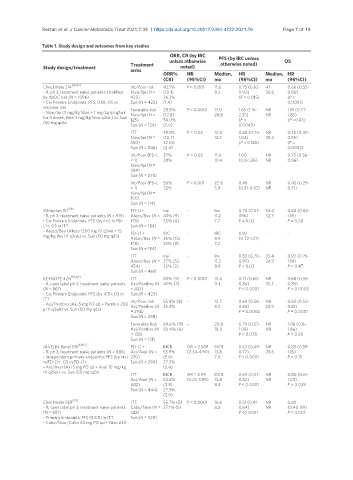
Table 1. Study design and outcomes from key studies
ORR, CR (by IRC PFS (by IRC unless
unless otherwise otherwise noted) OS
Study design/treatment Treatment noted)
arms
ORR% HR Median, HR Median, HR
(CR) (95%CI) mo (95%CI) mo (95%CI)
[16,43]
Checkmate 214 Int/Poor risk 42.1% P < 0.001 11.6 0.75 (0.62- 47 0.66 (0.55-
- R, ph 3; treatment naïve patients stratified Nivo/Ipi (N = (10.1) 8.3 0.90) 26.6 0.80)
by IMDC risk (N = 1096) 425) 26.3% (P = 0.015) (P <
- Co-Primary Endpoints: PFS, ORR, OS in Sun (N = 422) (1.4) 0.0001)
int/poor risk Favorable risk 28.8% P < 0.0001 17.0 1.65 (1.16- NR 1.19 (0.77-
- Nivo/Ipi (3 mg/kg Nivo + 1 mg/kg Ipi q3wk Nivo/Ipi (N = (12.8) 28.8 2.35) NR 1.85)
for 4 doses, then 3 mg/kg Nivo q2wk) vs. Sun 125) 54.0% (P = (P =0.43)
(50 mg qd)a
Sun (N = 124) (5.6) 0.0049)
ITT 39.1% P = 0.02 12.4 0.88 (0.75- NR 0.72 (0.49-
Nivo/Ipi (N = (10.7) 12.3 1.04) 38.4 0.95)
550) 32.6% (P = 0.126) (P =
Sun (N = 546) (2.4) 0.0002)
Int/Poor (PD-L 37% P = 0.03 11.0 1.00 NR 0.73 (0.56-
< 1) 28% 10.4 (0.8-1.26) NR 0.96)
Nivo/Ipi (N =
284)
Sun (N = 278)
Int/Poor (PD-L 58% P < 0.001 22.8 0.46 NR 0.45 (0.29-
> 1) 22% 5.9 (0.31-0.67) NR 0.71)
Nivo/Ipi (N =
100)
Sun (N = 114)
[58]
IMmotion 151 PD-L1+ Inv - Inv 0.74 (0.57- 34.0 0.84 (0.62-
- R, ph 3; treatment naïve patients (N = 915) Atezo/Bev (N = 43% (9) 11.2 096) 32.7 1.15)
- Co-Primary Endpoints: PFS (by inv) in PD- 178) 35% (4) 7.7 P = 0.02 P = 0.28
L1+, OS in ITT Sun (N = 184)
- Atezo/Bev (Atezo 1200 mg IV q3wk + 15 PD-L1 + IRC - IRC 0.93 - -
mg/kg Bev IV q3wk) vs. Sun (50 mg qd)a
Atezo/Bev (N = 36% (15) 8.9 (0.72-1.21)
178) 33% (8) 7.2
Sun (N = 184)
ITT Inv - Inv 0.83 (0.70- 33.4 0.93 (0.76-
Atezo/Bev (N = 37% (5) 11.2 0.97) 34.9 1.14)
454) 33% (2) 8.4 P = 0.02 P = 0.47
Sun (N = 461)
[59,60]
KEYNOTE 426 ITT 60% (9) P < 0.0001 15.4 0.71 (0.60- NR 0.68 (0.55-
- R, open label ph 3; treatment naïve patients Axi/Pembro (N 40% (3) 11.1 0.84) 35.7 0.85)
(N = 861) = 432) P < 0.0001 P = 0.0003
- Co-Primary Endpoints: PFS (by ICR), OS in Sun (N = 429)
ITT Int/Poor risk 55.8% (8) - 12.7 0.69 (0.56- NR 0.63 (0.50-
- Axi/Pembro (Axi 5 mg PO qd + Pembro 200 Axi/Pembro (N 35.2% 8.3 0.84) 28.9 0.81)
g IV q3wk) vs. Sun (50 mg qd)a
= 294) P = 0.0002 P = 0.0001
Sun (N = 298)
Favorable Risk 69.6% (11) - 20.8 0.79 (0.57- NR 1.06 (0.6-
Axi/Pembro (N 50.4% (6) 18.0 1.09) NR 1.86)
= 138) P = 0.078 P = 0.58
Sun (N = 131)
[61,62]
JAVELIN Renal 101 PD-L1 + BICR OR = 3.389 BICR 0.62 (0.49- NR 0.83 (0.59-
- R, ph 3; treatment naïve patients (N = 886) Axi/Avel (N = 55.9% (2.34-4.90) 13.8 0.77) 28.6 1.15)
- Independent primary endpoints: PFS (by inv) 270) (5.6) 7.0 P < 0.0001 P = 0.13
in PD-L1+, OS in PD-L1+ Sun (N = 290) 27.2%
- Axi/Avel (Axi 5 mg PO qd + Avel 10 mg/kg (2.4)
IV q2wk+ vs. Sun (50 mg qd)a
ITT BICR OR = 2.99 BICR 0.69 (0.57- NR 0.80 (0.61-
Axi/Avel (N = 52.5% (2.23-3.99) 13.8 0.82) NR 1.02)
442) (3.8) 8.4 P < 0.0001 P = 0.039
Sun (N = 444) 27.3%
(2.0)
[63]
Checkmate 9ER ITT 55.7% (8) P < 0.0001 16.6 0.51 (0.41- NR 0.60
- R, open label ph 3; treatment naïve patients Cabo/Nivo (N = 27.1% (5) 8.3 0.64) NR (0.40-89)
(N = 651) 323) P <0.0001 P = 0.001
- Primary endpoints: PFS (BICR) in ITT Sun (N = 328)
- Cabo/Nivo (Cabo 40 mg PO qd + Nivo 240