Page 24 - Read Online
P. 24
Mansinho et al. J Cancer Metastasis Treat 2021;7:44 https://dx.doi.org/10.20517/2394-4722.2021.88 Page 5 of 12
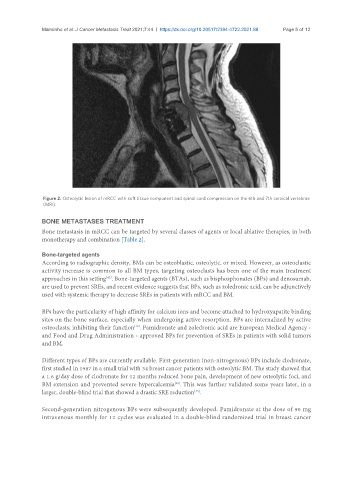
Figure 2. Osteolytic lesion of mRCC with soft tissue component and spinal cord compression on the 6th and 7th cervical vertebrae
(MRI).
BONE METASTASES TREATMENT
Bone metastasis in mRCC can be targeted by several classes of agents or local ablative therapies, in both
monotherapy and combination [Table 2].
Bone-targeted agents
According to radiographic density, BMs can be osteoblastic, osteolytic, or mixed. However, as osteoclastic
activity increase is common to all BM types, targeting osteoclasts has been one of the main treatment
approaches in this setting . Bone-targeted agents (BTAs), such as bisphosphonates (BPs) and denosumab,
[42]
are used to prevent SREs, and recent evidence suggests that BPs, such as zoledronic acid, can be adjunctively
used with systemic therapy to decrease SREs in patients with mRCC and BM.
BPs have the particularity of high affinity for calcium ions and become attached to hydroxyapatite binding
sites on the bone surface, especially when undergoing active resorption. BPs are internalized by active
osteoclasts, inhibiting their function . Pamidronate and zoledronic acid are European Medical Agency -
[43]
and Food and Drug Administration - approved BPs for prevention of SREs in patients with solid tumors
and BM.
Different types of BPs are currently available. First-generation (non-nitrogenous) BPs include clodronate,
first studied in 1987 in a small trial with 34 breast cancer patients with osteolytic BM. The study showed that
a 1.6 g/day dose of clodronate for 12 months reduced bone pain, development of new osteolytic foci, and
BM extension and prevented severe hypercalcemia . This was further validated some years later, in a
[44]
[45]
larger, double-blind trial that showed a drastic SRE reduction .
Second-generation nitrogenous BPs were subsequently developed. Pamidronate at the dose of 90 mg
intravenous monthly for 12 cycles was evaluated in a double-blind randomized trial in breast cancer