Page 84 - Read Online
P. 84
Lo Re et al. J Cancer Metastasis Treat 2020;6:17 I http://dx.doi.org/10.20517/2394-4722.2020.11 Page 3 of 9
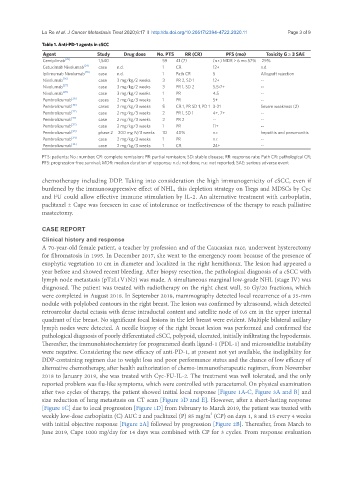
Table 1. Anti-PD-1 agents in cSCC
Agent Study Drug dose No. PTS RR (CR) PFS (mo) Toxicity G ≥ 3 SAE
Cemiplimab [23] 1,540 59 41 (7) (n.r.) MDR > 6 mo 57% 29%
Cetuximab Nivolumab [24] case n.d. 1 CR 12+ n.d
Ipilimumab Nivolumab [25] case n.d. 1 Path CR 5 Allograft rejection
Nivolumab [26] case 3 mg/kg/2 weeks 3 PR 2, SD 1 12+ --
Nivolumab [27] case 3 mg/kg/2 weeks 3 PR 1, SD 2 5.5-7+ --
Nivolumab [28] case 3 mg/kg/2 weeks 1 PR 4.5 --
Pembrolizumab [29] cases 2 mg/kg/3 weeks 1 PR 5+ --
Pembrolizumab [30] cases 2 mg/kg/3 weeks 5 CR 1, PR SD 1, PD 1 3-21 Severe weakness (2)
Pembrolizumab [27] case 2 mg/kg/3 weeks 2 PR 1, SD 1 4+, 7+ --
Pembrolizumab [31] case 2 mg/kg/3 weeks 2 PR 2 -- --
Pembrolizumab [32] case 2 mg/kg/3 weeks 1 PR 11+ --
Pembrolizumab [33] phase 2 200 mg IV/3 weeks 10 40% n.r. hepatitis and pneumonitis
Pembrolizumab [34] case 2 mg/kg/3 weeks 1 PR n.r. --
Pembrolizumab [35] case 2 mg/kg/3 weeks 1 CR 24+ --
PTS: patients; No.: number; CR: complete remission; PR: partial remission; SD: stable disease; RR: response rate; Path CR: pathological CR;
PFS: progression-free survival; MDR: median duration of response; n.d.: not done; n.r.: not reported; SAE: serious adverse event
chemotherapy including DDP. Taking into consideration the high immunogenicity of cSCC, even if
burdened by the immunosuppressive effect of NHL, this depletion strategy on Tregs and MDSCs by Cyc
and FU could allow effective immune stimulation by IL-2. An alternative treatment with carboplatin,
paclitaxel ± Cape was foreseen in case of intolerance or ineffectiveness of the therapy to reach palliative
mastectomy.
CASE REPORT
Clinical history and response
A 70-year-old female patient, a teacher by profession and of the Caucasian race, underwent hysterectomy
for fibromatosis in 1995. In December 2017, she went to the emergency room because of the presence of
exophytic vegetation 10 cm in diameter and localized in the right hemithorax. The lesion had appeared a
year before and showed recent bleeding. After biopsy resection, the pathological diagnosis of a cSCC with
lymph node metastasis (pT2L1V1N2) was made. A simultaneous marginal low-grade NHL (stage IV) was
diagnosed. The patient was treated with radiotherapy on the right chest wall, 50 Gy/20 fractions, which
were completed in August 2018. In September 2018, mammography detected local recurrence of a 35-mm
nodule with polylobed contours in the right breast. The lesion was confirmed by ultrasound, which detected
retroareolar ductal ectasia with dense intraductal content and satellite node of 0.6 cm in the upper internal
quadrant of the breast. No significant focal lesions in the left breast were evident. Multiple bilateral axillary
lymph nodes were detected. A needle biopsy of the right breast lesion was performed and confirmed the
pathological diagnosis of poorly differentiated cSCC, polypoid, ulcerated, initially infiltrating the hypodermis.
Thereafter, the immunohistochemistry for programmed death ligand-1 (PDL-1) and microsatellite instability
were negative. Considering the new efficacy of anti-PD-1, at present not yet available, the ineligibility for
DDP-containing regimen due to weight loss and poor performance status and the chance of low efficacy of
alternative chemotherapy, after health authorization of chemo-immunotherapeutic regimen, from November
2018 to January 2019, she was treated with Cyc-FU-IL-2. The treatment was well tolerated, and the only
reported problem was flu-like symptoms, which were controlled with paracetamol. On physical examination
after two cycles of therapy, the patient showed initial local response [Figure 1A-C, Figure 3A and B] and
size reduction of lung metastasis on CT scan [Figure 3D and E]. However, after a short-lasting response
[Figure 1C] due to local progression [Figure 1D] from February to March 2019, the patient was treated with
2
weekly low-dose carboplatin (C) AUC 2 and paclitaxel (P) 85 mg/m (CP) on days 1, 8 and 15 every 4 weeks
with initial objective response [Figure 2A] followed by progression [Figure 2B]. Thereafter, from March to
June 2019, Cape 1000 mg/day for 14 days was combined with CP for 3 cycles. From response evaluation