Page 86 - Read Online
P. 86
Lo Re et al. J Cancer Metastasis Treat 2020;6:17 I http://dx.doi.org/10.20517/2394-4722.2020.11 Page 5 of 9
A B C
D E F
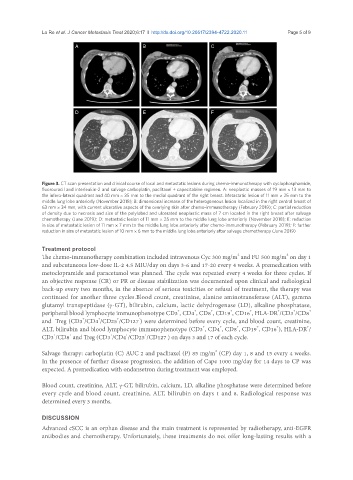
Figure 3. CT scan presentation and clinical course of local and metastatic lesions during chemo-immunotherapy with cyclophosphamide,
fluorouracil and interleukin-2 and salvage carboplatin, paclitaxel ± capecitabine regimen. A: neoplastic masses of 19 mm × 13 mm to
the infero-lateral quadrant and 40 mm × 35 mm to the medial quadrant of the right breast. Metastatic lesion of 11 mm × 25 mm to the
middle lung lobe anteriorly (November 2018); B: dimensional increase of the heterogeneous lesion localized in the right central breast of
63 mm × 34 mm, with current ulcerative aspects of the overlying skin after chemo-immunotherapy (February 2019); C: partial reduction
of density due to necrosis and size of the polylobed and ulcerated neoplastic mass of 7 cm located in the right breast after salvage
chemotherapy (June 2019); D: metastatic lesion of 11 mm × 25 mm to the middle lung lobe anteriorly (November 2018); E: reduction
in size of metastatic lesion of 11 mm × 7 mm to the middle lung lobe anteriorly after chemo-immunotherapy (February 2019); F: further
reduction in size of metastatic lesion of 10 mm × 6 mm to the middle lung lobe anteriorly after salvage chemotherapy (June 2019)
Treatment protocol
2
2
The chemo-immunotherapy combination included intravenous Cyc 300 mg/m and FU 500 mg/m on day 1
and subcutaneous low-dose IL-2 4.5 MIU/day on days 3-6 and 17-20 every 4 weeks. A premedication with
metoclopramide and paracetamol was planned. The cycle was repeated every 4 weeks for three cycles. If
an objective response (CR) or PR or disease stabilization was documented upon clinical and radiological
back-up every two months, in the absence of serious toxicities or refusal of treatment, the therapy was
continued for another three cycles.Blood count, creatinine, alanine aminotransferase (ALT), gamma
glutamyl transpeptidase (γ-GT), bilirubin, calcium, lactic dehydrogenase (LD), alkaline phosphatase,
+
+
+
+
+
+
+
peripheral blood lymphocyte immunophenotype CD3 , CD4 , CD8 , CD19 , CD16 , HLA-DR /CD3 /CD8
+
+
+
-
+
and Treg (CD3 /CD4 /CD25 /CD127 ) were determined before every cycle, and blood count, creatinine,
+
+
+
+
+
ALT, bilirubin and blood lymphocyte immunophenotype (CD3 , CD4 , CD8 , CD19 , CD16 ), HLA-DR /
+
+
+
+
+
+
-
CD3 /CD8 and Treg (CD3 /CD4 /CD25 /CD127 ) on days 3 and 17 of each cycle.
2
Salvage therapy: carboplatin (C) AUC 2 and paclitaxel (P) 85 mg/m (CP) day 1, 8 and 15 every 4 weeks.
In the presence of further disease progression, the addition of Cape 1000 mg/day for 14 days to CP was
expected. A premedication with ondansetron during treatment was employed.
Blood count, creatinine, ALT, γ-GT, bilirubin, calcium, LD, alkaline phosphatase were determined before
every cycle and blood count, creatinine, ALT, bilirubin on days 1 and 8. Radiological response was
determined every 3 months.
DISCUSSION
Advanced cSCC is an orphan disease and the main treatment is represented by radiotherapy, anti-EGFR
antibodies and chemotherapy. Unfortunately, these treatments do not offer long-lasting results with a