Page 113 - Read Online
P. 113
Page 6 of 20 Vidoni et al. J Cancer Metastasis Treat 2021;7:4 I http://dx.doi.org/10.20517/2394-4722.2020.95
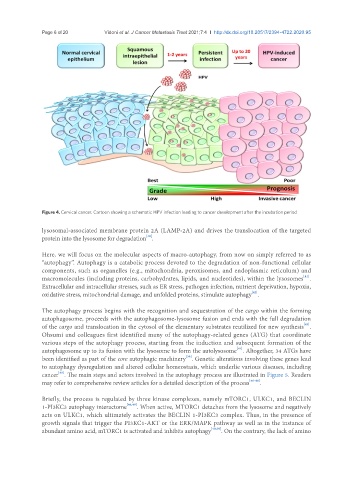
Figure 4. Cervical cancer. Cartoon showing a schematic HPV infection leading to cancer development after the incubation period
lysosomal-associated membrane protein 2A (LAMP-2A) and drives the translocation of the targeted
[40]
protein into the lysosome for degradation .
Here, we will focus on the molecular aspects of macro-autophagy, from now on simply referred to as
“autophagy”. Autophagy is a catabolic process devoted to the degradation of non-functional cellular
components, such as organelles (e.g., mitochondria, peroxisomes, and endoplasmic reticulum) and
[41]
macromolecules (including proteins, carbohydrates, lipids, and nucleotides), within the lysosomes .
Extracellular and intracellular stresses, such as ER stress, pathogen infection, nutrient deprivation, hypoxia,
[42]
oxidative stress, mitochondrial damage, and unfolded proteins, stimulate autophagy .
The autophagy process begins with the recognition and sequestration of the cargo within the forming
autophagosome, proceeds with the autophagosome-lysosome fusion and ends with the full degradation
[43]
of the cargo and translocation in the cytosol of the elementary substrates reutilized for new synthesis .
Ohsumi and colleagues first identified many of the autophagy-related genes (ATG) that coordinate
various steps of the autophagy process, starting from the induction and subsequent formation of the
[44]
autophagosome up to its fusion with the lysosome to form the autolysosome . Altogether, 34 ATGs have
[39]
been identified as part of the core autophagic machinery . Genetic alterations involving these genes lead
to autophagy dysregulation and altered cellular homeostasis, which underlie various diseases, including
cancer . The main steps and actors involved in the autophagy process are illustrated in Figure 5. Readers
[45]
may refer to comprehensive review articles for a detailed description of the process [46-48] .
Briefly, the process is regulated by three kinase complexes, namely mTORC1, ULKC1, and BECLIN
1-PI3KC3 autophagy interactome [46,49] . When active, MTORC1 detaches from the lysosome and negatively
acts on ULKC1, which ultimately activates the BECLIN 1-PI3KC3 complex. Thus, in the presence of
growth signals that trigger the PI3KC1-AKT or the ERK/MAPK pathway as well as in the instance of
abundant amino acid, mTORC1 is activated and inhibits autophagy [49,50] . On the contrary, the lack of amino