Page 431 - Read Online
P. 431
Klaas et al. J Cancer Metastasis Treat 2023;9:23 https://dx.doi.org/10.20517/2394-4722.2022.125 Page 5 of 15
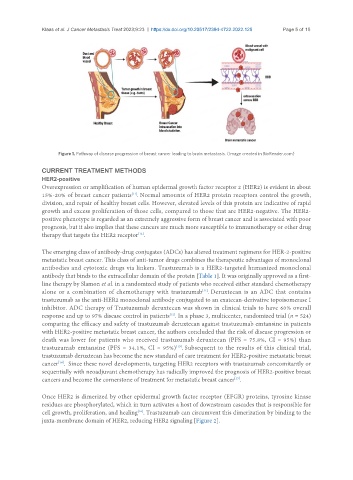
Figure 1. Pathway of disease progression of breast cancer leading to brain metastasis. (Image created in BioRender.com)
CURRENT TREATMENT METHODS
HER2-positive
Overexpression or amplification of human epidermal growth factor receptor 2 (HER2) is evident in about
[11]
15%-20% of breast cancer patients . Normal amounts of HER2 protein receptors control the growth,
division, and repair of healthy breast cells. However, elevated levels of this protein are indicative of rapid
growth and excess proliferation of those cells, compared to those that are HER2-negative. The HER2-
positive phenotype is regarded as an extremely aggressive form of breast cancer and is associated with poor
prognosis, but it also implies that these cancers are much more susceptible to immunotherapy or other drug
therapy that targets the HER2 receptor .
[12]
The emerging class of antibody-drug conjugates (ADCs) has altered treatment regimens for HER-2-positive
metastatic breast cancer. This class of anti-tumor drugs combines the therapeutic advantages of monoclonal
antibodies and cytotoxic drugs via linkers. Trastuzumab is a HER2-targeted humanized monoclonal
antibody that binds to the extracellular domain of the protein [Table 1]. It was originally approved as a first-
line therapy by Slamon et al. in a randomized study of patients who received either standard chemotherapy
[11]
alone or a combination of chemotherapy with trastuzumab . Deruxtecan is an ADC that contains
trastuzumab as the anti-HER2 monoclonal antibody conjugated to an exatecan-derivative topoisomerase I
inhibitor. ADC therapy of Trastuzumab deruxtecan was shown in clinical trials to have 60% overall
response and up to 97% disease control in patients . In a phase 3, multicenter, randomized trial (n = 524)
[12]
comparing the efficacy and safety of trastuzumab deruxtecan against trastuzumab emtansine in patients
with HER2-positive metastatic breast cancer, the authors concluded that the risk of disease progression or
death was lower for patients who received trastuzumab deruxtecan (PFS = 75.8%, CI = 95%) than
trastuzumab emtansine (PFS = 34.1%, CI = 95%) . Subsequent to the results of this clinical trial,
[13]
trastuzumab deruxtecan has become the new standard of care treatment for HER2-positive metastatic breast
[14]
cancer . Since these novel developments, targeting HER2 receptors with trastuzumab concomitantly or
sequentially with neoadjuvant chemotherapy has radically improved the prognosis of HER2-positive breast
cancers and become the cornerstone of treatment for metastatic breast cancer .
[15]
Once HER2 is dimerized by other epidermal growth factor receptor (EFGR) proteins, tyrosine kinase
residues are phosphorylated, which in turn activates a host of downstream cascades that is responsible for
cell growth, proliferation, and healing . Trastuzumab can circumvent this dimerization by binding to the
[16]
juxta-membrane domain of HER2, reducing HER2 signaling [Figure 2].