Page 430 - Read Online
P. 430
Page 4 of 15 Klaas et al. J Cancer Metastasis Treat 2023;9:23 https://dx.doi.org/10.20517/2394-4722.2022.125
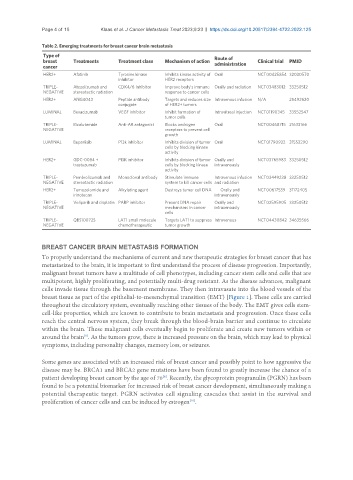
Table 2. Emerging treatments for breast cancer brain metastasis
Type of Route of
breast Treatments Treatment class Mechanism of action administration Clinical trial PMID
cancer
HER2+ Afatinib Tyrosine kinase Inhibits kinase activity of Oral NCT00425854 32030570
inhibitor HER2 receptors
TRIPLE- Atezolizumab and CDK4/6 Inhibitor Improve body’s immune Orally and radiation NCT03483012 33250512
NEGATIVE stereotactic radiation response to cancer cells
HER2+ ANG4043 Peptide antibody Targets and reduces size Intravenous infusion N/A 25492620
conjugate of HER2+ tumors
LUMINAL Bevacizumab VEGF inhibitor Inhibit formation of Intravitreal injection NCT01190345 33552547
tumor cells
TRIPLE- Bicalutamide Anti-AR antagonist Blocks androgen Oral NCT00468715 21633166
NEGATIVE receptors to prevent cell
growth
LUMINAL Buparlisib P13k inhibitor Inhibits division of tumor Oral NCT01790932 31552290
cells by blocking kinase
activity
HER2+ GDC-0084 + PI3K inhibitor Inhibits division of tumor Orally and NCT03765983 33250512
trastuzumab cells by blocking kinase intravenously
activity
TRIPLE- Pembrolizumab and Monoclonal antibody Stimulate immune Intravenous infusion NCT03449238 33250512
NEGATIVE stereotactic radiation system to kill cancer cells and radiation
HER2+ Temozolomide and Alkylating agent Destroys tumor cell DNA Orally and NCT00617539 31172405
irinotecan intravenously
TRIPLE- Veliparib and cisplatin PARP inhibitor Prevent DNA repair Orally and NCT02595905 33250512
NEGATIVE mechanisms in cancer intravenously
cells
TRIPLE- QBS10072S LAT1 small molecule Targets LAT1 to suppress Intravenous NCT04430842 34635566
NEGATIVE chemotherapeutic tumor growth
BREAST CANCER BRAIN METASTASIS FORMATION
To properly understand the mechanisms of current and new therapeutic strategies for breast cancer that has
metastasized to the brain, it is important to first understand the process of disease progression. Importantly,
malignant breast tumors have a multitude of cell phenotypes, including cancer stem cells and cells that are
multipotent, highly proliferating, and potentially multi-drug resistant. As the disease advances, malignant
cells invade tissue through the basement membrane. They then intravasate into the blood vessels of the
breast tissue as part of the epithelial-to-mesenchymal transition (EMT) [Figure 1]. These cells are carried
throughout the circulatory system, eventually reaching other tissues of the body. The EMT gives cells stem-
cell-like properties, which are known to contribute to brain metastasis and progression. Once these cells
reach the central nervous system, they break through the blood-brain barrier and continue to circulate
within the brain. These malignant cells eventually begin to proliferate and create new tumors within or
[8]
around the brain . As the tumors grow, there is increased pressure on the brain, which may lead to physical
symptoms, including personality changes, memory loss, or seizures.
Some genes are associated with an increased risk of breast cancer and possibly point to how aggressive the
disease may be. BRCA1 and BRCA2 gene mutations have been found to greatly increase the chance of a
patient developing breast cancer by the age of 70 . Recently, the glycoprotein progranulin (PGRN) has been
[9]
found to be a potential biomarker for increased risk of breast cancer development, simultaneously making a
potential therapeutic target. PGRN activates cell signaling cascades that assist in the survival and
[10]
proliferation of cancer cells and can be induced by estrogen .