Page 436 - Read Online
P. 436
Page 10 of 15 Klaas et al. J Cancer Metastasis Treat 2023;9:23 https://dx.doi.org/10.20517/2394-4722.2022.125
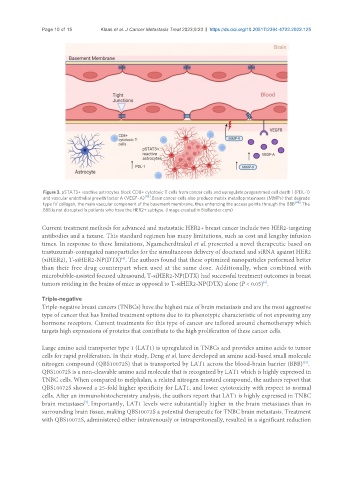
Figure 3. pSTAT3+ reactive astrocytes block CD8+ cytotoxic T cells from cancer cells and upregulate programmed cell death 1 (PDL-1)
and vascular endothelial growth factor A (VEGF-A) [42] . Brain cancer cells also produce matrix metalloproteinases (MMPs) that degrade
type IV collagen, the main vascular component of the basement membrane, thus enhancing the access points through the BBB [46] . The
BBB is not disrupted in patients who have the HER2+ subtype. (Image created in BioRender.com)
Current treatment methods for advanced and metastatic HER2+ breast cancer include two HER2-targeting
antibodies and a taxane. This standard regimen has many limitations, such as cost and lengthy infusion
times. In response to these limitations, Ngamcherdtrakul et al. presented a novel therapeutic based on
trastuzumab-conjugated nanoparticles for the simultaneous delivery of docetaxel and siRNA against HER2
(siHER2), T-siHER2-NP(DTX) . The authors found that these optimized nanoparticles performed better
[6]
than their free drug counterpart when used at the same dose. Additionally, when combined with
microbubble-assisted focused ultrasound, T-siHER2-NP(DTX) had successful treatment outcomes in breast
[6]
tumors residing in the brains of mice as opposed to T-siHER2-NP(DTX) alone (P < 0.05) .
Triple-negative
Triple-negative breast cancers (TNBCs) have the highest rate of brain metastasis and are the most aggressive
type of cancer that has limited treatment options due to its phenotypic characteristic of not expressing any
hormone receptors. Current treatments for this type of cancer are tailored around chemotherapy which
targets high expressions of proteins that contribute to the high proliferation of these cancer cells.
Large amino acid transporter type 1 (LAT1) is upregulated in TNBCs and provides amino acids to tumor
cells for rapid proliferation. In their study, Deng et al. have developed an amino acid-based small molecule
nitrogen compound (QBS10072S) that is transported by LAT1 across the blood-brain barrier (BBB) .
[51]
QBS10072S is a non-cleavable amino acid molecule that is recognized by LAT1 which is highly expressed in
TNBC cells. When compared to melphalan, a related nitrogen mustard compound, the authors report that
QBS10072S showed a 25-fold higher specificity for LAT1, and lower cytotoxicity with respect to normal
cells. After an immunohistochemistry analysis, the authors report that LAT1 is highly expressed in TNBC
brain metastases . Importantly, LAT1 levels were substantially higher in the brain metastases than in
[7]
surrounding brain tissue, making QBS10072S a potential therapeutic for TNBC brain metastasis. Treatment
with QBS10072S, administered either intravenously or intraperitoneally, resulted in a significant reduction