Page 413 - Read Online
P. 413
Droste et al. J Cancer Metastasis Treat 2023;9:2 https://dx.doi.org/10.20517/2394-4722.2022.94 Page 9 of 22
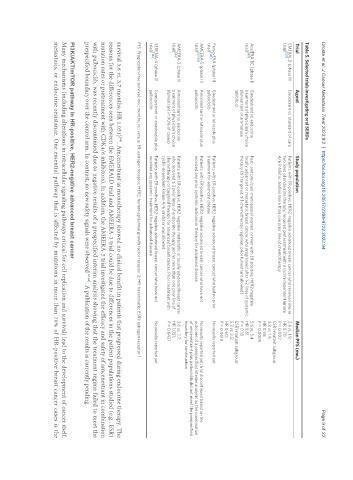
Table 5. Selected trials investigating oral SERDs
Trial Agent Study population Median PFS (mo.)
EMERALD (phase III Elacestrant vs. standard of care Patients with ER-positive, HER2-negative advanced breast cancer who received one or 2.8 vs. 1.9;
[59]
trial) two lines of endocrine therapy; required pretreatment with a cyclin-dependent kinase HR 0.70;
4/6 inhibitor, and no more than one prior line of chemotherapy P = 0.002
ESR1-mutant subgroup:
3.8 vs. 1.9;
HR 0.55;
P = 0.0005
AcelERA BC (phase II Giredestrant vs. endocrine Post- and pre-/peri-menopausal women, or men with ER-positive, HER2-negative 5.6 vs. 5.4
[63]
trial) treatment of physician’s choice locally advanced or metastatic breast cancer who progressed after 1-2 lines of systemic HR 0.81
(fulvestrant or aromatase therapy (≤ 1 targeted, ≤ 1 chemotherapy regimen, prior fulvestrant allowed) P = 0.18
inhibitor) ESR1-mutant subgroup:
5.3 vs. 3.5;
HR 0.60;
P = 0.0610
PersevERA (phase III Giredestrant or letrozole plus Patients with ER-positive, HER2-negative advanced breast cancer who had no prior No results reported yet
[141]
trial) palbociclib treatment for advanced disease
AMEERA-5 (phase III Amcenestrant or letrozole plus Patients with ER-positive, HER2-negative advanced breast cancer who have not No results reported yet; trial discontinued based on the
trial) [67,68] palbociclib received any prior systemic anticancer therapy for advanced disease outcome of a prespecified interim analysis as the combination
of amcenestrant plus palbociclib did not meet the prespecified
boundary for continuation
AMEERA-3 (phase II Amcenestrant vs. endocrine Patients with ER-positive, HER2-negative metastatic or locally advanced breast cancer 3.6 vs. 3.7;
[64]
trial) treatment of physician’s choice who received ≤ 2 prior lines of endocrine therapy and no more than one prior line of HR 1.051;
(fulvestrant in 90% of cases) chemotherapy or targeted therapy for advanced breast cancer. Prior treatment with P = 0.6437
cyclin-dependent kinase 4/6 inhibitor was allowed.
SERENA-4 (phase III Camizestrant or anastrozole plus Patients with ER-positive, HER2-negative advanced breast cancer who have not No results reported yet
[142]
trial) palbociclib received any systemic treatment for advanced disease
PFS: Progression-free survival; mo.: months; vs.: versus; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; ESR1: estrogen receptor 1.
survival 3.6 vs. 3.7 months; HR 1.051) . Amcenestrant as monotherapy showed no clinical benefit in patients that progressed during endocrine therapy. The
[64]
reasons for the differences seen between the EMERALD trial and AMEERA-3 trial could be due to differences in the patient populations studied (e.g., ESR1
mutation rates or pretreatment with CDK4/6 inhibitors). In addition, the AMEERA-5 trial investigated the efficacy and safety of amcenestrant in combination
with palbociclib, was recently discontinued due to negative results of a prespecified interim analysis showing that the treatment regime failed to meet the
prespecified boundary over the control arm. In contrast, no new safety signals were observed [67,68] . A publication of the results is currently pending.
PI3K/AKT/mTOR pathway in HR-positive, HER2-negative advanced breast cancer
Many mechanisms (including alterations in intracellular signaling pathways critical for cell replication and survival) lead to the development of cancer itself,
metastasis, or endocrine resistance. One essential pathway that is affected by mutations in more than 70% of HR-positive breast cancer cases is the