Page 409 - Read Online
P. 409
Droste et al. J Cancer Metastasis Treat 2023;9:2 https://dx.doi.org/10.20517/2394-4722.2022.94 Page 5 of 22
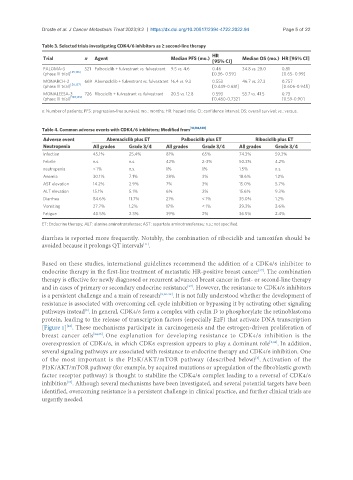
Table 3. Selected trials investigating CDK4/6 inhibitors as ≥ second-line therapy
HR
Trial n Agent Median PFS (mo.) Median OS (mo.) HR [95% CI]
[95% CI]
PALOMA-3 521 Palbociclib + fulvestrant vs. fulvestrant 9.5 vs. 4.6 0.46 34.8 vs. 28.0 0.81
(phase III trial) [25,136] [0.36- 0.59] [0.65- 0.99]
MONARCH-2 669 Abemaclclib + fulvestrant vs. fulvestrant 16.4 vs. 9.3 0.553 46.7 vs. 37.3 0.757
(phase III trial) [26,137] [0.449-0.681] [0.606-0.945]
MONALEESA-3 726 Ribociclib + fulvestrant vs. fulvestrant 20.5 vs. 12.8 0.593 53.7 vs. 41.5 0.73
[138,139]
(phase III trial) [0.480-0.732] [0.59-0.90]
n: Number of patients; PFS: progression-free survival; mo.: months; HR: hazard ratio; CI: confidence interval; OS: overall survival; vs.: versus.
Table 4. Common adverse events with CDK4/6 inhibitors; Modified from [13,136,140]
Adverse event Abemaciclib plus ET Palbociclib plus ET Ribociclib plus ET
Neutropenia All grades Grade 3/4 All grades Grade 3/4 All grades Grade 3/4
Infection 45.1% 25.4% 81% 65% 74.3% 59.3%
Febrile n.s. n.s. 42% 2-3% 50.3% 4.2%
neutropenia < 1% n.s. 1% 1% 1.5% n.s.
Anemia 30.1% 7.1% 28% 3% 18.6% 1.2%
AST elevation 14.2% 2.9% 7% 3% 15.0% 5.7%
ALT elevation 15.1% 5.1% 6% 2% 15.6% 9.3%
Diarrhea 84.6% 11.7% 21% < 1% 35.0% 1.2%
Vomiting 27.7% 1.2% 17% < 1% 29.3% 3.6%
Fatigue 40.5% 2.3% 39% 2% 36.5% 2.4%
ET: Endocrine therapy; ALT: alanine aminotransferase; AST: aspartate aminotransferase; n.s.: not specified.
diarrhea is reported more frequently. Notably, the combination of ribociclib and tamoxifen should be
avoided because it prolongs QT intervals .
[11]
Based on these studies, international guidelines recommend the addition of a CDK4/6 inhibitor to
[27]
endocrine therapy in the first-line treatment of metastatic HR-positive breast cancer . The combination
therapy is effective for newly diagnosed or recurrent advanced breast cancer in first- or second-line therapy
and in cases of primary or secondary endocrine resistance . However, the resistance to CDK4/6 inhibitors
[27]
is a persistent challenge and a main of research [5,30-32] . It is not fully understood whether the development of
resistance is associated with overcoming cell cycle inhibition or bypassing it by activating other signaling
pathways instead . In general, CDK4/6 form a complex with cyclin D to phosphorylate the retinoblastoma
[5]
protein, leading to the release of transcription factors (especially E2F) that activate DNA transcription
[Figure 1] . These mechanisms participate in carcinogenesis and the estrogen-driven proliferation of
[33]
breast cancer cells [34,35] . One explanation for developing resistance to CDK4/6 inhibition is the
overexpression of CDK4/6, in which CDK6 expression appears to play a dominant role [5,36] . In addition,
several signaling pathways are associated with resistance to endocrine therapy and CDK4/6 inhibition. One
of the most important is the PI3K/AKT/mTOR pathway (described below) . Activation of the
[5]
PI3K/AKT/mTOR pathway (for example, by acquired mutations or upregulation of the fibroblastic growth
factor receptor pathway) is thought to stabilize the CDK4/6 complex leading to a reversal of CDK4/6
[37]
inhibition . Although several mechanisms have been investigated, and several potential targets have been
identified, overcoming resistance is a persistent challenge in clinical practice, and further clinical trials are
urgently needed.