Page 407 - Read Online
P. 407
Droste et al. J Cancer Metastasis Treat 2023;9:2 https://dx.doi.org/10.20517/2394-4722.2022.94 Page 3 of 22
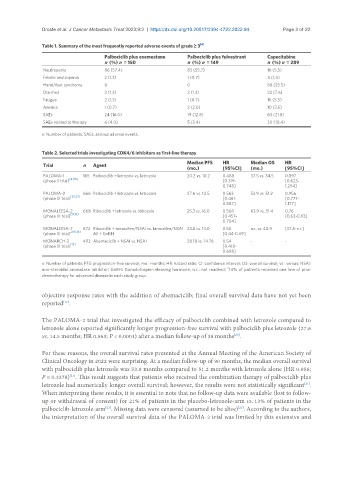
Table 1. Summary of the most frequently reported adverse events of grade ≥ 3 [6]
Palbociclib plus exemestane Palbociclib plus fulvestrant Capecitabine
n (%) n = 150 n (%) n = 149 n (%) n = 289
Neutropenia 86 (57.4) 83 (55.7) 16 (5.5)
Febrile neutropenia 2 (1.3) 1 (0.7) 4 (1.4)
Hand/foot syndrome 0 0 68 (23.5)
Diarrhea 2 (1.3) 2 (1.3) 22 (7.6)
Fatigue 2 (1.3) 1 (0.7) 16 (5.5)
Anemia 1 (0.7) 3 (2.0) 10 (3.5)
SAEs 24 (16.0) 19 (12.8) 63 (21.8)
SAEs related to therapy 6 (4.0) 5 (3.4) 30 (10.4)
n: Number of patients; SAEs: serious adverse events.
Table 2. Selected trials investigating CDK4/6 inhibitors as first-line therapy
Median PFS HR Median OS HR
Trial n Agent
(mo.) [95%CI] (mo.) [95%CI]
PALOMA-1 165 Palbociclib + letrozole vs. letrozole 20.2 vs. 10.2 0.488 37.5 vs. 34.5 0.897
(phase II trial) [8,135] [0.319- [0.623-
0.748] 1.294]
PALOMA-2 666 Palbociclib + letrozole vs. letrozole 27.6 vs. 14.5 0.563 53.9 vs. 51.2 0.956
[20,21]
(phase III trial) [0.461- [0.777-
0.687] 1.177]
MONALEESA-2 668 Ribociclib + letrozole vs. letrozole 25.3 vs. 16.0 0.568 63.9 vs. 51.4 0.76
(phase III trial) [11,18] [0.457- [0.63-0.93]
0.704]
MONALEESA-7 672 Ribociclib + tamoxifen/NSAI vs. tamoxifen/NSAI 23.8 vs. 13.0 0.55 n.r. vs. 40.9 [37.8-n.r.]
(phase III trial) a[10,14] All + GnRH [0.44-0.69]
MONARCH-3 493 Abemaciclib + NSAI vs. NSAI 28.18 vs. 14.76 0.54 - -
(phase III trial) [12] [0.418-
0.698]
n: Number of patients; PFS: progression-free survival; mo.: months; HR: hazard ratio; CI: confidence interval; OS: overall survival; vs.: versus; NSAI:
a
non-steroidal aromatase inhibitor; GnRH: Gonadotropin-releasing hormone; n.r.: not reached; 14% of patients received one line of prior
chemotherapy for advanced disease in each study group.
objective response rates with the addition of abemaciclib; final overall survival data have not yet been
[12]
reported .
The PALOMA-2 trial that investigated the efficacy of palbociclib combined with letrozole compared to
letrozole alone reported significantly longer progression-free survival with palbociclib plus letrozole (27.6
vs. 14.5 months; HR 0.563; P < 0.0001) after a median follow-up of 38 months .
[20]
For these reasons, the overall survival rates presented at the Annual Meeting of the American Society of
Clinical Oncology in 2022 were surprising. At a median follow-up of 90 months, the median overall survival
with palbociclib plus letrozole was 53.9 months compared to 51.2 months with letrozole alone (HR 0.956;
[21]
P = 0.3378) . This result suggests that patients who received the combination therapy of palbociclib plus
letrozole had numerically longer overall survival; however, the results were not statistically significant .
[21]
When interpreting these results, it is essential to note that no follow-up data were available (lost to follow-
up or withdrawal of consent) for 21% of patients in the placebo-letrozole-arm vs. 13% of patients in the
[21]
palbociclib-letrozole-arm . Missing data were censored (assumed to be alive) . According to the authors,
[21]
the interpretation of the overall survival data of the PALOMA-2 trial was limited by this extensive and