Page 410 - Read Online
P. 410
Page 6 of 22 Droste et al. J Cancer Metastasis Treat 2023;9:2 https://dx.doi.org/10.20517/2394-4722.2022.94
[5]
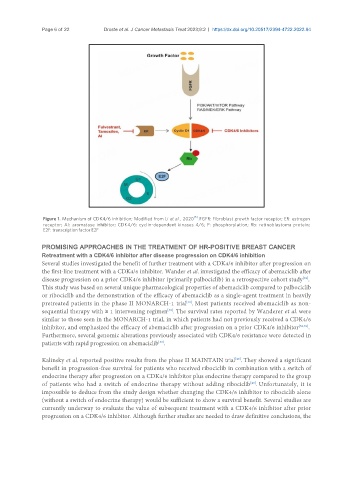
Figure 1. Mechanism of CDK4/6 inhibition; Modified from Li et al., 2020 . FGFR: Fibroblast growth factor receptor; ER: estrogen
receptor; AI: aromatase inhibitor; CDK4/6: cyclin-dependent kinases 4/6; P: phosphorylation; Rb: retinoblastoma protein;
E2F: transcription factor E2F
PROMISING APPROACHES IN THE TREATMENT OF HR-POSITIVE BREAST CANCER
Retreatment with a CDK4/6 inhibitor after disease progression on CDK4/6 inhibition
Several studies investigated the benefit of further treatment with a CDK4/6 inhibitor after progression on
the first-line treatment with a CDK4/6 inhibitor. Wander et al. investigated the efficacy of abemaciclib after
[38]
disease progression on a prior CDK4/6 inhibitor (primarily palbociclib) in a retrospective cohort study .
This study was based on several unique pharmacological properties of abemaciclib compared to palbociclib
or ribociclib and the demonstration of the efficacy of abemaciclib as a single-agent treatment in heavily
[39]
pretreated patients in the phase II MONARCH-1 trial . Most patients received abemaciclib as non-
sequential therapy with ≥ 1 intervening regimen . The survival rates reported by Wanderer et al. were
[38]
similar to those seen in the MONARCH-1 trial, in which patients had not previously received a CDK4/6
inhibitor, and emphasized the efficacy of abemaciclib after progression on a prior CDK4/6 inhibitor [38,39] .
Furthermore, several genomic alterations previously associated with CDK4/6 resistance were detected in
patients with rapid progression on abemaciclib .
[38]
[40]
Kalinsky et al. reported positive results from the phase II MAINTAIN trial . They showed a significant
benefit in progression-free survival for patients who received ribociclib in combination with a switch of
endocrine therapy after progression on a CDK4/6 inhibitor plus endocrine therapy compared to the group
of patients who had a switch of endocrine therapy without adding ribociclib . Unfortunately, it is
[40]
impossible to deduce from the study design whether changing the CDK4/6 inhibitor to ribociclib alone
(without a switch of endocrine therapy) would be sufficient to show a survival benefit. Several studies are
currently underway to evaluate the value of subsequent treatment with a CDK4/6 inhibitor after prior
progression on a CDK4/6 inhibitor. Although further studies are needed to draw definitive conclusions, the