Page 211 - Read Online
P. 211
dos Santos et al. J Cancer Metastasis Treat 2019;5:25 I http://dx.doi.org/10.20517/2394-4722.2018.83 Page 3 of 20
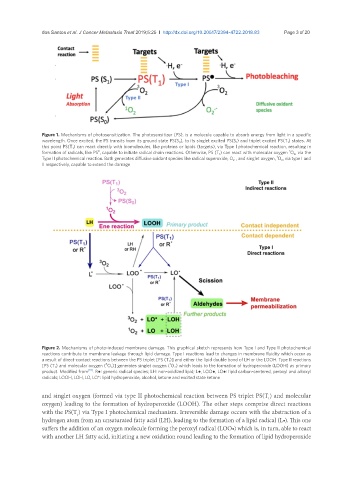
Figure 1. Mechanisms of photosensitization. The photosensitizer (PS), is a molecule capable to absorb energy from light in a specific
wavelength. Once excited, the PS transits from its ground state PS(S 0 ), to its singlet excited PS(S 1 ) and triplet excited PS(T 1 ) states. At
this point PS(T 1 ) can react directly with biomolecules, like proteins or lipids (targets), via Type I photochemical reaction, resulting in
3
●
formation of radicals, like PS , capable to initiate radical chain reactions. Otherwise, PS (T 1 ) can react with molecular oxygen O 2 , via the
.-
1
Type II photochemical reaction. Both generates diffusive oxidant species like radical superoxide, O 2 , and singlet oxygen, O 2 , via type I and
II respectively, capable to extend the damage
Figure 2. Mechanisms of photo-induced membrane damage. This graphical sketch represents how Type I and Type II photochemical
reactions contribute to membrane leakage through lipid damage. Type I reactions lead to changes in membrane fluidity which occur as
a result of direct-contact reactions between the PS triplet [PS (T 1 )] and either the lipid double bond of LH or the LOOH. Type II reactions
1
3
[PS (T 1 ) and molecular oxygen ( O 2 )] generates singlet oxygen ( O 2 ) which leads to the formation of hydroperoxide (LOOH) as primary
product. Modified from [18] . R●: generic radical species; LH: non-oxidized lipid; L●, LOO●, LO●: lipid carbon-centered, peroxyl and alkoxyl
radicals; LOOH, LOH, LO, LO*: lipid hydroperoxide, alcohol, ketone and excited state ketone
and singlet oxygen (formed via type II photochemical reaction between PS triplet PS(T ) and molecular
1
oxygen) leading to the formation of hydroperoxide (LOOH). The other steps comprise direct reactions
with the PS(T ) via Type I photochemical mechanism. Irreversible damage occurs with the abstraction of a
1
hydrogen atom from an unsaturated fatty acid (LH), leading to the formation of a lipid radical (L•). This one
suffers the addition of an oxygen molecule forming the peroxyl radical (LOO•) which is, in turn, able to react
with another LH fatty acid, initiating a new oxidation round leading to the formation of lipid hydroperoxide