Page 12 - Read Online
P. 12
Page 6 of 17 Spallanzani et al. J Cancer Metastasis Treat 2018;4:28 I http://dx.doi.org/10.20517/2394-4722.2018.31
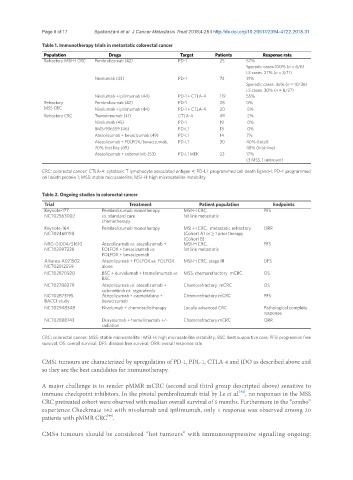
Table 1. Immunotherapy trials in metastatic colorectal cancer
Population Drugs Target Patients Response rate
Refractory MSI-H CRC Pembrolizumab (42) PD-1 25 57%
Sporadic cases:100% (n = 6/6)
LS cases: 27% (n = 3/11)
Nivolumab (43) PD-1 74 31%
Sporadic cases: 36% (n = 10/36)
LS cases: 30% (n = 8/27)
Nivolumab + ipilimumab (44) PD-1 + CTLA-4 119 55%
Refractory Pembrolizumab (42) PD-1 28 0%
MSS CRC Nivolumab + ipilimumab (44) PD-1 + CTLA-4 20 5%
Refractory CRC Tremelimumab (41) CTLA-4 49 2%
Nivolumab (45) PD-1 19 0%
BMS-936559 (46) PD-L1 18 0%
Atezolizumab + bevacizumab (49) PD-L1 14 7%
Atezolizumab + FOLFOX/bevacizumab, PD-L1 30 40% (total)
70% first line (49) 48% (first-line)
Atezolizumab + cobimetinib (53) PD-L1 MEK 23 17%
(3 MSS, 1 unknown)
CRC: colorectal cancer; CTLA-4: cytotoxic T lymphocyte associated antigen 4; PD-L1: programmed cell death ligand-1; PD-1: programmed
cell death protein 1; MSS: stable microsatellite; MSI-H: high microsatellite instability
Table 2. Ongoing studies in colorectal cancer
Trial Treatment Patient population Endpoints
Keynote-177 Pembrolizumab monotherapy MSI-H CRC, PFS
NCT02563002 vs. standard care 1st line metastatic
chemotherapy
Keynote-164 Pembrolizumab monotherapy MSI-H CRC, metastatic refractory ORR
NCT02460198 (Cohort A) or ≥ 1 prior therapy
(Cohort B)
NRG-GI004/S1610 Atezolizumab vs. atezolizumab + MSI-H CRC, PFS
NCT02997228 FOLFOX + bevacizumab vs. 1st line metastatic
FOLFOX + bevacizumab
Alliance A021502 Atezolizumab + FOLFOX vs. FOLFOX MSI-H CRC, stage III DFS
NCT02912559 alone
NCT02870920 BSC + durvalumab + tremelimumab vs. MSS, chemorefractory mCRC OS
BSC
NCT02788279 Atezolizumab vs. atezolizumab + Chemorefractory mCRC OS
cobimetinib vs. regorafenib
NCT02873195 Atezolizumab + capecitabina + Chemorefractory mCRC PFS
BACCI study bevacizumab
NCT02948348 Nivolumab + chemoradiotherapy Locally advanced CRC Pathological complete
response
NCT02888743 Durvalumab + tremelimumab +/- Chemorefractory mCRC ORR
radiation
CRC: colorectal cancer; MSS: stable microsatellite ; MSI-H: high microsatellite instability; BSC: best supportive care; PFS: progression free
survival; OS: overall survival; DFS: disease free survival; ORR: overall response rate
CMS1 tumours are characterized by upregulation of PD-1, PDL-1, CTLA-4 and IDO as described above and
so they are the best candidates for immunotherapy.
A major challenge is to render pMMR mCRC (second and third group descripted above) sensitive to
[42]
immune checkpoint inhibitors. In the pivotal pembrolizumab trial by Le et al. , no responses in the MSS
CRC pretreated cohort were observed with median overall survival of 5 months. Furthermore in the “combo”
experience Checkmate 142 with nivolumab and ipilimumab, only 1 response was observed among 20
[44]
patients with pMMR CRC .
CMS4 tumours should be considered “hot tumours” with immunosuppressive signalling ongoing: