Page 43 - Read Online
P. 43
Yagishita et al. J Cancer Metastasis Treat 2019;5:75 I http://dx.doi.org/10.20517/2394-4722.2019.026 Page 9 of 13
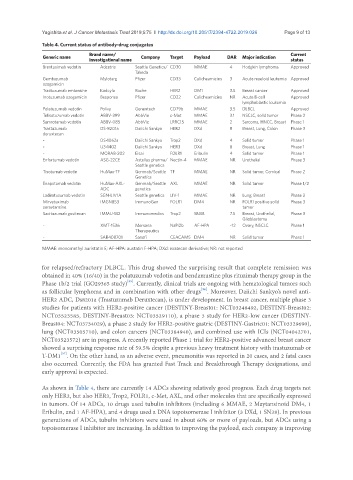
Table 4. Current status of antibody-drug conjugates
Brand name/ Current
Generic name Company Target Payload DAR Major indication
investigational name status
Brentuximab vedotin Adcetris Seattle Genetics/ CD30 MMAE 4 Hodgkin lymphoma Approved
Takeda
Gemtuzumab Mylotarg Pfizer CD33 Calicheamicins 3 Acute myeloid leukemia Approved
ozogamicin
Trastuzumab emtansine Kadcyla Roche HER2 DM1 3.5 Breast cancer Approved
Inotuzumab ozogamicin Besponsa Pfizer CD22 Calicheamicins NR Acute B-cell Approved
lymphoblastic leukemia
Polatuzumab vedotin Polivy Genentech CD79b MMAE 3.5 DLBCL Approved
Telisotuzumab vedotin ABBV-399 AbbVie c-Met MMAE 3.1 NSCLC, solid tumor Phase 2
Samrotamab vedotin ABBV-085 AbbVie LRRC15 MMAE 2 Sarcoma, HNCC, Breast Phase 1
Trastuzumab DS-8201a Daiichi Sankyo HER2 DXd 8 Breast, Lung, Colon Phase 3
deruxtecan
- DS-1062a Daiichi Sankyo Trop2 DXd 4 Solid tumor Phase 1
- U3-1402 Daiichi Sankyo HER3 DXd 8 Breast, Lung Phase 1
- MORAB-202 Eisai FOLR1 Eribulin 4 Solid tumor Phase 1
Enfortumab vedotin ASG-22CE Astellas pharma/ Nectin-4 MMAE NR Urothelial Phase 3
Seattle genetics
Tisotumab vedotin HuMax-TF Genmab/Seattle TF MMAE NR Solid tumor, Cervical Phase 2
Genetics
Enapotamab vedotin HuMax-AXL- Genmab/Seattle AXL MMAE NR Solid tumor Phase 1/2
ADC genetics
Ladiratuzumab vedotin SGN-LIV1A Seattle genetics LIV-1 MMAE NR Lung, Breast Phase 2
Mirvetuximab IMGN853 ImmunoGen FOLR1 DM4 NR FOLR1 positive solid Phase 3
soravtansine tumor
Sacituzumab govitecan IMMU-132 Immunomedics Trop2 SN38 7.5 Breast, Urothelial, Phase 3
Glioblastoma
- XMT-1536 Mersana NaPi2b AF-HPA ~12 Ovary, NSCLC Phase 1
Therapeutics
- SAR408701 Sanofi CEACAM5 DM4 NR Solid tumor Phase 1
MMAE: monomethyl auristatin E; AF-HPA: austatin F-HPA; DXd: exatecan derivative; NR: not reported
for relapsed/refractory DLBCL. This drug showed the surprising result that complete remission was
obtained in 40% (16/40) in the polatuzumab vedotin and bendamustine plus rituzimab therapy group in the
Phase 1b/2 trial (GO29365 study) . Currently, clinical trials are ongoing with hematological tumors such
[55]
[56]
as follicular lymphoma and in combination with other drugs . Moreover, Daiichi Sankyo’s novel anti-
HER2 ADC, Ds8201a (Trastuzumab Deruxtecan), is under development. In breast cancer, multiple phase 3
studies for patients with HER2-positive cancer (DESTINY-Breast01: NCT03248492, DESTINY-Breast02:
NCT03523585, DESTINY-Breast03: NCT03529110), a phase 3 study for HER2-low cancer (DESTINY-
Breast04: NCT03734029), a phase 2 study for HER2-positive gastric (DESTINY-Gastric01: NCT03329690),
lung (NCT03505710), and colon cancers (NCT03384940), and combined use with ICIs (NCT04042701,
NCT03523572) are in progress. A recently reported Phase 1 trial for HER2-positive advanced breast cancer
showed a surprising response rate of 59.5% despite a previous heavy treatment history with trastuzumab or
[57]
T-DM1 . On the other hand, as an adverse event, pneumonitis was reported in 20 cases, and 2 fatal cases
also occurred. Currently, the FDA has granted Fast Track and Breakthrough Therapy designations, and
early approval is expected.
As shown in Table 4, there are currently 14 ADCs showing relatively good progress. Each drug targets not
only HER2, but also HER3, Trop2, FOLR1, c-Met, AXL, and other molecules that are specifically expressed
in tumors. Of 14 ADCs, 10 drugs used tubulin inhibitors (including 6 MMAE, 2 Maytansinoid DM4, 1
Eribulin, and 1 AF-HPA), and 4 drugs used a DNA topoisomerase I inhibitor (3 DXd, 1 SN38). In previous
generations of ADCs, tubulin inhibitors were used in about 60% or more of payloads, but ADCs using a
topoisomerase I inhibitor are increasing. In addition to improving the payload, each company is improving