Page 39 - Read Online
P. 39
Yagishita et al. J Cancer Metastasis Treat 2019;5:75 I http://dx.doi.org/10.20517/2394-4722.2019.026 Page 5 of 13
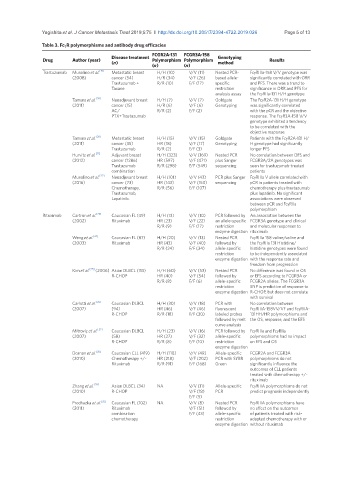
Table 3. FcgR polymorphisms and antibody drug efficacies
FCGR2A-131 FCGR3A-158
Disease treatment Genotyping
Drug Author (year) Polymorphism Polymorphism Results
(n) method
(n) (n)
Trastuzumab Musolino et al. [19] Metastatic breast H/H (10) V/V (11) Nested PCR- FcgRIIIa-158 V/V genotype was
(2008) cancer (54) H/R (34) V/F (26) based allele- significantly correlated with ORR
Trastuzumab + R/R (10) F/F (17) specific and PFS. There was a trend to
Taxane restriction significance in ORR and PFS for
analysis assay the FcgRIIa-131 H/H genotype
Tamura et al. [20] Neoadjuvant breast H/H (7) V/V (7) Goldgate The FcgR2A-131 H/H genotype
(2011) cancer (15) H/R (6) V/F (6) Genotyping was significantly correlated
AC/ R/R (2) F/F (2) with the pCR and the objective
PTX+Trastuzumab response. The FcgR3A-158 V/V
genotype exhibited a tendency
to be correlated with the
objective response
Tamura et al. [20] Metastatic breast H/H (15) V/V (15) Goldgate Patients with the FcgR2A-131 H/
(2011) cancer (35) HR (18) V/F (17) Genotyping H genotype had significantly
Trastuzumab R/R (2) F/F (3) longer PFS
Hurvitz et al. [21] Adjuvant breast H/H (323) V/V (169) Nested PCR No correlation between DFS and
(2012) cancer (1286) HR (597) V/F (471) plus Sanger FCGR3A/2A genotypes was
Trastuzumab R/R (298) F/F (549) sequencing seen for trastuzumab-treated
combination patients
Musolino et al. [22] Neoadjuvant breast H/H (101) V/V (43) PCR plus Sanger FcgRIIIa V allele correlated with
(2016) cancer (73) HR (143) V/F (150) sequencing pCR in patients treated with
Chemotherapy, R/R (56) F/F (107) chemotherapy plus trastuzumab
Trastuzumab, plus lapatinib. No significant
Lapatinib. associations were observed
between pCR and FcgRIIa
polymorphism
Rituximab Cartron et al. [23] Caucasian FL (49) H/H (13) V/V (10) PCR followed by An association between the
(2002) Rituximab HR (23) V/F (22) an allele-specific FCGR3A genotype and clinical
R/R (9) F/F (17) restriction and molecular responses to
enzyme digestion rituximab
Weng et al. [24] Caucasian FL (87) H/H (20) V/V (13) Nested PCR FcgRIIIa 158 valine/valine and
(2003) Rituximab HR (43) V/F (40) followed by the FcgRIIa 131 Histidine/
R/R (24) F/F (34) allele-specific histidine genotypes were found
restriction to be independently associated
enzyme digestion with the response rate and
freedom from progression
Kim et al. [25] (2006) Asian DLBCL (113) H/H (60) V/V (53) Nested PCR No difference was found in OS
R-CHOP HR (40) V/F (54) followed by or EFS according to FCGR3A or
R/R (8) F/F (6) allele-specific FCGR2A alleles. The FCGR3A
restriction SNP is predictive of response to
enzyme digestion R-CHOP, but does not correlate
with survival
Carlotti et al. [26] Caucasian DLBCL H/H (30) V/V (18) PCR with No correlation between
(2007) (94) HR (46) V/F (46) fluorescent FcgRIIIA-158VV/VF and FcgRIIA-
R-CHOP R/R (18) F/F (30) labeled probes 131HH/HR polymorphisms and
followed by melt the OS, response, and the EFS
curve analysis
Mitroviç et al. [27] Caucasian DLBCL H/H (23) V/V (16) PCR followed by FcgRIIIa and FcgRIIa
(2007) (58) HR (27) V/F (32) allele-specific polymorphisms had no impact
R-CHOP R/R (8) F/F (10) restriction on EFS and OS
enzyme digestion
Dornan et al. [28] Caucasian CLL (419) H/H (110) V/V (49) Allele-specific FCGR2A and FCGR3A
(2010) Chemotherapy +/- HR (218) V/F (202) PCR with SYBR polymorphisms do not
Rituximab R/R (91) F/F (168) Green significantly influence the
outcomes of CLL patients
treated with chemotherapy +/-
rituximab
Zhang et al. [29] Asian DLBCL (34) NA V/V (11) Allele-specific FcgRIIIA polymorphisms do not
(2010) R-CHOP V/F (18) PCR predict prognosis independently
F/F (5)
Prochazka et al. [32] Caucasian FL (102) NA V/V (8) Nested PCR FcgRIIIA polymorphisms have
(2011) Rituximab V/F (51) followed by no effect on the outcomes
combination F/F (43) allele-specific of patients treated with risk-
chemotherapy restriction adapted chemotherapy with or
enzyme digestion without rituximab