Page 53 - Read Online
P. 53
Yagawa et al. Cancer immunity and hyperthermia
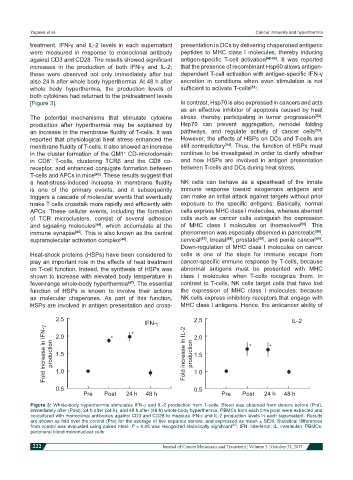
treatment. IFN-γ and IL-2 levels in each supernatant presentation in DCs by delivering chaperoned antigenic
were measured in response to monoclonal antibody peptides to MHC class I molecules, thereby inducing
against CD3 and CD28. The results showed significant antigen-specific T-cell activation [48-50] . It was reported
increases in the production of both IFN-γ and IL-2; that the presence of recombinant Hsp60 allows antigen-
these were observed not only immediately after but dependent T-cell activation with antigen-specific IFN-γ
also 24 h after whole body hyperthermia. At 48 h after secretion in conditions when even stimulation is not
whole body hyperthermia, the production levels of sufficient to activate T-cells [51] .
both cytokines had returned to the pretreatment levels
[Figure 3]. In contrast, Hsp70 is also expressed in cancers and acts
as an effective inhibitor of apoptosis caused by heat
The potential mechanisms that stimulate cytokine stress, thereby participating in tumor progression [52] .
production after hyperthermia may be explained by Hsp70 can prevent aggregation, remodel folding
an increase in the membrane fluidity of T-cells. It was pathways, and regulate activity of cancer cells [53] .
reported that physiological heat stress enhanced the However, the effects of HSPs on DCs and T-cells are
membrane fluidity of T-cells. It also showed an increase still contradictory [54] . Thus, the function of HSPs must
in the cluster formation of the GM1 CD-microdomain continue to be investigated in order to clarify whether
+
in CD8 T-cells, clustering TCRβ and the CD8 co- and how HSPs are involved in antigen presentation
+
receptor, and enhanced conjugate formation between between T-cells and DCs during heat stress.
T-cells and APCs in mice [43] . These results suggest that
a heat-stress-induced increase in membrane fluidity NK cells can behave as a spearhead of the innate
is one of the primary events, and it subsequently immune response toward exogenous antigens and
triggers a cascade of molecular events that eventually can make an initial attack against targets without prior
make T cells crosstalk more rapidly and efficiently with exposure to the specific antigens. Basically, normal
APCs. These cellular events, including the formation cells express MHC class I molecules, whereas aberrant
of TCR microclusters, consist of several adhesion cells such as cancer cells extinguish the expression
and signaling molecules [44] , which accumulate at the of MHC class I molecules on themselves [55] . This
immune synapse [45] . This is also known as the central phenomenon was especially observed in pancreatic [56] ,
supramolecular activation complex [46] . cervical [57] , breast [58] , prostatic [59] , and penile cancer [60] .
Down-regulation of MHC class I molecules on cancer
Heat-shock proteins (HSPs) have been considered to cells is one of the steps for immune escape from
play an important role in the effects of heat treatment cancer-specific immune response by T-cells, because
on T-cell function. Indeed, the synthesis of HSPs was abnormal antigens must be presented with MHC
shown to increase with elevated body temperature in class I molecules when T-cells recognize them. In
fever-range whole-body hyperthermia [47] . The essential contrast to T-cells, NK cells target cells that have lost
function of HSPs is known to involve their actions the expression of MHC class I molecules, because
as molecular chaperones. As part of this function, NK cells express inhibitory receptors that engage with
HSPs are involved in antigen presentation and cross- MHC class I antigens. Hence, the anticancer ability of
2.5 IFN-g 2.5 IL-2
Fold increase in IFN-g production 2.0 Fold increase in IL-2 production 2.0
1.5
1.5
1.0
0.5 1.0
0.5
Pre Post 24 h 48 h Pre Post 24 h 48 h
Figure 3: Whole-body hyperthermia stimulates IFN-g and IL-2 production from T-cells. Blood was obtained from donors before (Pre),
immediately after (Post), 24 h after (24 h), and 48 h after (48 h) whole-body hyperthermia. PBMCs from each time point were extracted and
co-cultured with monoclonal antibodies against CD3 and CD28 to measure IFN-g and IL-2 production levels in each supernatant. Results
are shown as fold over the control (Pre) for the average of five separate donors, and expressed as mean ± SEM. Statistical differences
[42]
from control was evaluated using paired t-test. P < 0.05 was recognized statistically significant . IFN: interferon; IL: interleukin; PBMCs:
peripheral blood mononuclear cells
222 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ October 31, 2017