Page 52 - Read Online
P. 52
Yagawa et al. Cancer immunity and hyperthermia
The more recent discovery of immune check-point section, we discuss how thermal stress up-regulates
inhibitors achieved outstanding progress in cancer the immune system.
immunotherapy by showing sensational long-
term benefits in patients with advanced cancer [4,5] . Hyperthermia, especially whole body hyperthermia,
The purpose of this medicine is to inhibit immune has the potential to increase the homing of immune
suppressive signals between cancer cells and T-cells; cells. Continuous secretion of homeostatic chemokines
thus the agent that eliminates a cancer during the final including CCL21 and the expression of adhesion
phase is T-cells [36,37] . Immune check-point molecules factors including selectin, integrin, and ICAM regulate
such as programmed death-1 (PD-1) and T-lymphocyte- immune homeostasis by maintaining the homing of
associated antigen 4 (CTLA4) are expressed on T these immune cells. Thermal effect can enhance the
cells and play a vital role in limiting the exaggerated expression of ICAM-1 and CCL21 in high endothelial
immune response in both adaptive immune response venules (HEVs) [39] and can up-regulate L-selectin- and
and autoimmune response to maintain homeostasis by integrin-dependent adhesive interaction to induce the
acting as an inhibitory signal against APCs. Recently, adhesion and migration of DCs and T-cells toward
it has been disclosed that cancer cells take advantage HEVs [40] . Additionally, increases in the migration
of this mechanism to survive. For example, cancer capacity of DCs ex vivo has been reported [41] .
cells express PD-L1, which is a concomitant ligand
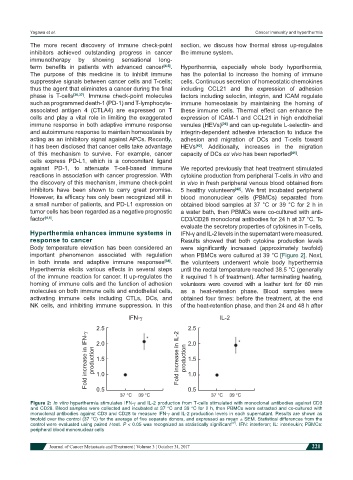
against PD-1, to attenuate T-cell-based immune We reported previously that heat treatment stimulated
reactions in association with cancer progression. With cytokine production from peripheral T-cells in vitro and
the discovery of this mechanism, immune check-point in vivo in fresh peripheral venous blood obtained from
inhibitors have been shown to carry great promise. 5 healthy volunteers [42] . We first incubated peripheral
However, its efficacy has only been recognized still in blood mononuclear cells (PBMCs) separated from
a small number of patients, and PD-L1 expression on obtained blood samples at 37 °C or 39 °C for 2 h in
tumor cells has been regarded as a negative prognostic a water bath, then PBMCs were co-cultured with anti-
factor [4,5] . CD3/CD28 monoclonal antibodies for 24 h at 37 °C. To
evaluate the secretory properties of cytokines in T-cells,
Hyperthermia enhances immune systems in IFN-γ and IL-2 levels in the supernatant were measured.
response to cancer Results showed that both cytokine production levels
Body temperature elevation has been considered an were significantly increased (approximately twofold)
important phenomenon associated with regulation when PBMCs were cultured at 39 °C [Figure 2]. Next,
in both innate and adaptive immune responses [38] . the volunteers underwent whole body hyperthermia
Hyperthermia elicits various effects in several steps until the rectal temperature reached 38.5 °C (generally
of the immune reaction for cancer. It up-regulates the it required 1 h of treatment). After terminating heating,
homing of immune cells and the function of adhesion volunteers were covered with a leather tent for 60 min
molecules on both immune cells and endothelial cells, as a heat-retention phase. Blood samples were
activating immune cells including CTLs, DCs, and obtained four times: before the treatment, at the end
NK cells, and inhibiting immune suppression. In this of the heat-retention phase, and then 24 and 48 h after
IFN-g IL-2
2.5 2.5
Fold increase in IFN-g production 1.5 Fold increase in IL-2 production 1.5
2.0
2.0
1.0
1.0
0.5
0.5
37 °C 39 °C 37 °C 39 °C
Figure 2: In vitro hyperthermia stimulates IFN-g and IL-2 production from T-cells stimulated with monoclonal antibodies against CD3
and CD28. Blood samples were collected and incubated at 37 °C and 39 °C for 2 h, then PBMCs were extracted and co-cultured with
monoclonal antibodies against CD3 and CD28 to measure IFN-g and IL-2 production levels in each supernatant. Results are shown as
twofold over the control (37 °C) for the average of five separate donors, and expressed as mean ± SEM. Statistical differences from the
[42]
control were evaluated using paired t-test. P < 0.05 was recognized as statistically significant . IFN: interferon; IL: interleukin; PBMCs:
peripheral blood mononuclear cells
Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ October 31, 2017 221