Page 17 - Read Online
P. 17
Umetsu et al. Hepatoma Res 2020;6:1 I http://dx.doi.org/10.20517/2394-5079.2019.030 Page 5 of 10
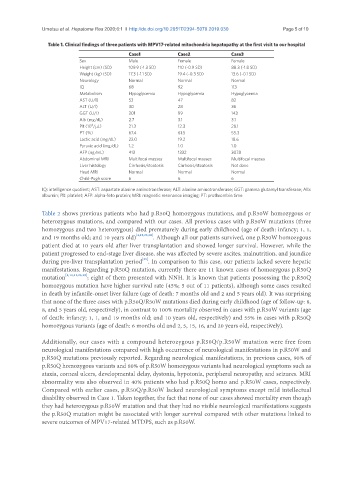
Table 1. Clinical findings of three patients with MPV17-related mitochondria hepatopathy at the first visit to our hospital
Case1 Case2 Case3
Sex Male Female Female
Height (cm) (SD) 109.9 (-1.3 SD) 110 (-0.9 SD) 88.3 (-1.8 SD)
Weight (kg) (SD) 17.3 (-1.1 SD) 19.4 (-0.3 SD) 13.6 (-0.1 SD)
Neurology Normal Normal Normal
IQ 68 92 113
Metabolism Hypoglycemia Hypoglycemia Hypoglycemia
AST (U/l) 53 47 82
ALT (U/l) 30 28 36
GGT (U/l) 201 99 143
Alb (mg/dL) 2.7 3.1 3.1
4
Plt (10 /μL) 21.3 12.3 28.1
PT (%) 67.4 61.5 55.3
Lactic acid (mg/dL) 22.0 19.2 18.6
Pyruvic acid (mg/dL) 1.2 1.0 1.0
AFP (ng/mL) 413 1332 3078
Abdominal MRI Multifocal masses Multifocal masses Multifocal masses
Liver histology Cirrhosis/steatosis Cirrhosis/steatosis Not done
Head MRI Normal Normal Normal
Child-Pugh score 6 6 6
IQ: intelligence quotient; AST: aspartate alanine aminotransferase; ALT: alanine aminotransferase; GGT: gamma glutamyl transferase; Alb:
albumin; Plt: platelet; AFP: alpha-feto protein; MRI: magnetic resonance imaging; PT: prothrombin time
Table 2 shows previous patients who had p.R50Q homozygous mutations, and p.R50W homozygous or
heterozygous mutations, and compared with our cases. All previous cases with p.R50W mutations (three
homozygous and two heterozygous) died prematurely during early childhood (age of death: infancy; 1, 1,
and 19 months old; and 10 years old) [4,12,25,30] . Although all our patients survived, one p.R50W homozygous
patient died at 10 years old after liver transplantation and showed longer survival. However, while the
patient progressed to end-stage liver disease, she was affected by severe ascites, malnutrition, and jaundice
during pre-liver transplantation period . In comparison to this case, our patients lacked severe hepatic
[25]
manifestations. Regarding p.R50Q mutation, currently there are 11 known cases of homozygous p.R50Q
mutation [4,10,11,25,29] ; eight of them presented with NNH. It is known that patients possessing the p.R50Q
homozygous mutation have higher survival rate (45%; 5 out of 11 patients), although some cases resulted
in death by infantile-onset liver failure (age of death: 7 months old and 2 and 5 years old). It was surprising
that none of the three cases with p.R50Q/R50W mutations died during early childhood (age of follow-up: 8,
8, and 5 years old, respectively), in contrast to 100% mortality observed in cases with p.R50W variants (age
of death: infancy; 1, 1, and 19 months old; and 10 years old, respectively) and 55% in cases with p.R50Q
homozygous variants (age of death: 6 months old and 2, 5, 15, 16, and 20 years old, respectively).
Additionally, our cases with a compound heterozygous p.R50Q/p.R50W mutation were free from
neurological manifestations compared with high occurrence of neurological manifestations in p.R50W and
p.R50Q mutations previously reported. Regarding neurological manifestations, in previous cases, 90% of
p.R50Q homozygous variants and 80% of p.R50W homozygous variants had neurological symptoms such as
ataxia, corneal ulcers, developmental delay, dystonia, hypotonia, peripheral neuropathy, and seizures. MRI
abnormality was also observed in 40% patients who had p.R50Q homo and p.R50W cases, respectively.
Compared with earlier cases, p.R50Q/p.R50W lacked neurological symptoms except mild intellectual
disability observed in Case 1. Taken together, the fact that none of our cases showed mortality even though
they had heterozygous p.R50W mutation and that they had no visible neurological manifestations suggests
the p.R50Q mutation might be associated with longer survival compared with other mutations linked to
severe outcomes of MPV17-related MTDPS, such as p.R50W.