Page 63 - Read Online
P. 63
Jiang et al. Hepatoma Res 2019;5:5 I http://dx.doi.org/10.20517/2394-5079.2018.97 Page 7 of 11
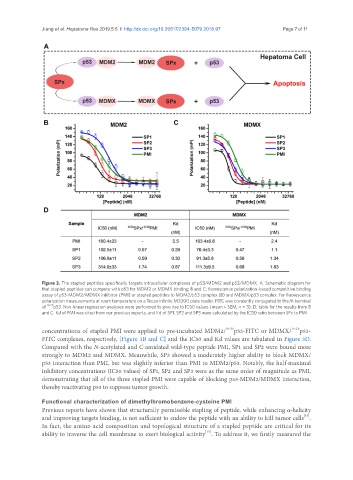
Figure 3. The stapled peptides specifically targets intracellular complexes of p53/MDM2 and p53/MDMX. A: Schematic diagram for
that stapled peptides can compete with p53 for MDM2 or MDMX binding; B and C: fluorescence polarization-based competitive binding
assay of p53-MDM2/MDMX inhibitor (PMI) or stapled peptides to MDM2/p53 complex (B) and MDMX/p53 complex. For fluorescence
polarization measurements at room temperature on a Tecan Infinite M2000 plate reader, FITC was covalently conjugated to the N-terminal
of 15-29 p53. Non-linear regression analyses were performed to give rise to IC50 values (mean ± SEM, n = 3); D: table for the results from B
and C. Kd of PMI was cited from our previous reports, and Kd of SP1, SP2 and SP3 were calculated by the IC50 ratio between SPx to PMI
concentrations of stapled PMI were applied to pre-incubated MDM2/ 18-26 p53-FITC or MDMX/ 18-26 p53-
FITC complexes, respectively, [Figure 3B and C] and the IC50 and Kd values are tabulated in Figure 3D.
Compared with the N-acetylated and C-amidated wild-type peptide PMI, SP1 and SP2 were bound more
strongly to MDM2 and MDMX. Meanwhile, SP3 showed a moderately higher ability to block MDMX/
p53 interaction than PMI, but was slightly inferior than PMI to MDM2/p53. Notably, the half-maximal
inhibitory concentrations (IC50 values) of SP1, SP2 and SP3 were as the same order of magnitude as PMI,
demonstrating that all of the three stapled PMI were capable of blocking p53-MDM2/MDMX interaction,
thereby reactivating p53 to suppress tumor growth.
Functional characterization of dimethylbromobenzene-cysteine PMI
Previous reports have shown that structurally permissible stapling of peptide, while enhancing α-helicity
[15]
and improving targets binding, is not sufficient to endow the peptide with an ability to kill tumor cells .
In fact, the amino acid composition and topological structure of a stapled peptide are critical for its
[15]
ability to traverse the cell membrane to exert biological activity . To address it, we firstly measured the