Page 62 - Read Online
P. 62
Page 6 of 11 Jiang et al. Hepatoma Res 2019;5:5 I http://dx.doi.org/10.20517/2394-5079.2018.97
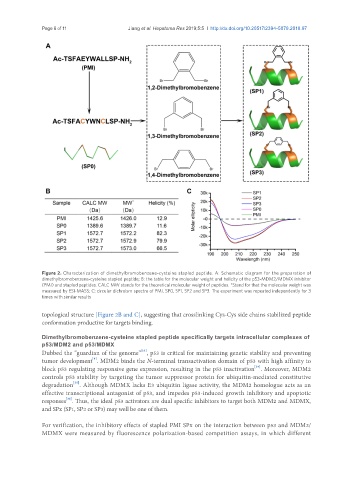
Figure 2. Characterization of dimethylbromobenzene-cysteine stapled peptide. A: Schematic diagram for the preparation of
dimethylbromobenzene-cysteine stapled peptide; B: the table for the molecular weight and helicity of the p53-MDM2/MDMX inhibitor
(PMI) and stapled peptides. CALC MW stands for the theoretical molecular weight of peptides. *Stand for that the molecular weight was
measured by ESI-MASS; C: circular dichroism spectra of PMI, SP0, SP1, SP2 and SP3. The experiment was repeated independently for 3
times with similar results
topological structure [Figure 2B and C], suggesting that crosslinking Cys-Cys side chains stabilized peptide
conformation productive for targets binding.
Dimethylbromobenzene-cysteine stapled peptide specifically targets intracellular complexes of
p53/MDM2 and p53/MDMX
[33]
Dubbed the “guardian of the genome” , p53 is critical for maintaining genetic stability and preventing
[4]
tumor development . MDM2 binds the N-terminal transactivation domain of p53 with high affinity to
[34]
block p53 regulating responsive gene expression, resulting in the p53 inactivation . Moreover, MDM2
controls p53 stability by targeting the tumor suppressor protein for ubiquitin-mediated constitutive
[35]
degradation . Although MDMX lacks E3 ubiquitin ligase activity, the MDM2 homologue acts as an
effective transcriptional antagonist of p53, and impedes p53-induced growth inhibitory and apoptotic
responses . Thus, the ideal p53 activators are dual specific inhibitors to target both MDM2 and MDMX,
[36]
and SPx (SP1, SP2 or SP3) may well be one of them.
For verification, the inhibitory effects of stapled PMI SPx on the interaction between p53 and MDM2/
MDMX were measured by fluorescence polarization-based competition assays, in which different