Page 194 - Read Online
P. 194
Page 6 of 11 Nakamura et al. Hepatoma Res 2019;5:16 I http://dx.doi.org/10.20517/2394-5079.2019.06
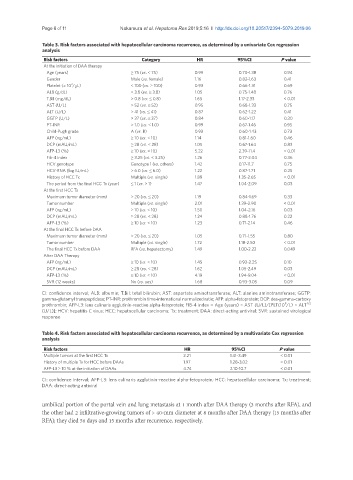
Table 3. Risk factors associated with hepatocellular carcinoma recurrence, as determined by a univariate Cox regression
analysis
Risk factors Category HR 95%CI P value
At the initiation of DAA therapy
Age (years) ≥ 75 (vs. < 75) 0.99 0.70-1.38 0.94
Gender Male (vs. female) 1.16 0.82-1.63 0.41
Platelet (× 10 /μL) < 100 (vs. ≥ 100) 0.93 0.66-1.31 0.69
3
ALB (g/dL) < 3.8 (vs. ≥ 3.8) 1.05 0.75-1.48 0.76
T.Bil (mg/dL) > 0.8 (vs. ≤ 0.8) 1.65 1.17-2.33 < 0.01
AST (U/L) > 52 (vs. ≤ 52) 0.95 0.68-1.33 0.75
ALT (U/L) > 41 (vs. ≤ 41) 0.87 0.62-1.22 0.41
GGTP (U/L) > 37 (vs. ≤ 37) 0.84 0.60-1.17 0.30
PT-INR > 1.0 (vs. ≤ 1.0) 0.99 0.67-1.46 0.95
Child-Pugh grade A (vs. B) 0.93 0.60-1.43 0.73
AFP (ng/mL) ≥ 10 (vs. < 10) 1.14 0.81-1.60 0.46
DCP (mAU/mL) ≥ 28 (vs. < 28) 1.05 0.67-1.64 0.83
AFP-L3 (%) ≥ 10 (vs. < 10) 5.22 2.39-11.4 < 0.01
Fib-4 index ≥ 3.25 (vs. < 3.25) 1.26 0.77-2.04 0.36
HCV genotype Genotype 1 (vs. others) 1.42 0.17-11.7 0.75
HCV-RNA (log IU/mL) > 6.0 (vs. ≤ 6.0) 1.22 0.87-1.71 0.25
History of HCC Tx Multiple (vs. single) 1.89 1.35-2.65 < 0.01
The period from the final HCC Tx (year) ≤ 1 (vs. > 1) 1.47 1.04-2.09 0.03
At the first HCC Tx
Maximum tumor diameter (mm) > 20 (vs. ≤ 20) 1.19 0.84-1.69 0.33
Tumor number Multiple (vs. single) 2.01 1.39-2.90 < 0.01
AFP (ng/mL) ≥ 10 (vs. < 10) 1.50 1.04-2.16 0.03
DCP (mAU/mL) ≥ 28 (vs. < 28) 1.24 0.88-1.76 0.22
AFP-L3 (%) ≥ 10 (vs. < 10) 1.23 0.71-2.14 0.46
At the final HCC Tx before DAA
Maximum tumor diameter (mm) > 20 (vs. ≤ 20) 1.05 0.71-1.55 0.80
Tumor number Multiple (vs. single) 1.72 1.18-2.50 < 0.01
The final HCC Tx before DAA RFA (vs. hepatectomy) 1.49 1.00-2.22 0.049
After DAA Therapy
AFP (ng/mL) ≥ 10 (vs. < 10) 1.45 0.93-2.25 0.10
DCP (mAU/mL) ≥ 28 (vs. < 28) 1.62 1.05-2.49 0.03
AFP-L3 (%) ≥ 10 (vs. < 10) 4.19 1.94-9.04 < 0.01
SVR (12 weeks) No (vs. yes) 1.68 0.93-3.05 0.09
CI: confidence interval; ALB: albumin; T.Bil: total bilirubin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGTP:
gamma-glutamyl transpeptidase; PT-INR: prothrombin time-international normalized ratio; AFP: alpha-fetoprotein; DCP: des-gamma-carboxy
1/2
9
prothrombin; AFP-L3: lens culinaris agglutinin-reactive alpha-fetoprotein; FIB-4 index = Age (years) × AST (U/L)/[PLT(10 /L) × ALT
(U/L)]; HCV: hepatitis C virus; HCC: hepatocellular carcinoma; Tx: treatment; DAA: direct-acting antiviral; SVR: sustained virological
response
Table 4. Risk factors associated with hepatocellular carcinoma recurrence, as determined by a multivariate Cox regression
analysis
Risk factors HR 95%CI P value
Multiple tumors at the first HCC Tx 2.21 1.41-3.49 < 0.01
History of multiple Tx for HCC before DAAs 1.97 1.28-3.02 < 0.01
AFP-L3 ≥ 10 % at the initiation of DAAs 4.74 2.10-10.7 < 0.01
CI: confidence interval; AFP-L3: lens culinaris agglutinin-reactive alpha-fetoprotein; HCC: hepatocellular carcinoma; Tx: treatment;
DAA: direct-acting antiviral
umbilical portion of the portal vein and lung metastasis at 1 month after DAA therapy (3 months after RFA), and
the other had 2 infiltrative-growing tumors of > 40-mm diameter at 8 months after DAA therapy (15 months after
RFA); they died 56 days and 15 months after recurrence, respectively.