Page 191 - Read Online
P. 191
Nakamura et al. Hepatoma Res 2019;5:16 I http://dx.doi.org/10.20517/2394-5079.2019.06 Page 3 of 11
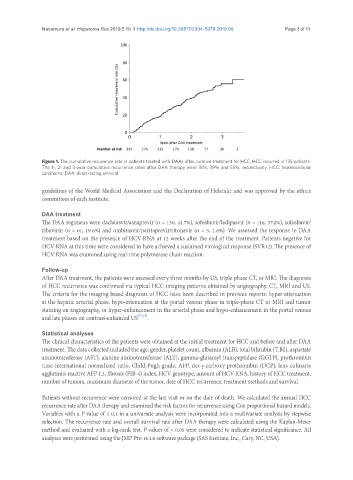
Figure 1. The cumulative recurrence rate in patients treated with DAAs after curative treatment for HCC. HCC recurred in 135 patients.
The 1-, 2- and 3-year cumulative recurrence rates after DAA therapy were 18%, 39% and 55%, respectively. HCC: hepatocellular
carcinoma; DAA: direct-acting antiviral
guidelines of the World Medical Association and the Declaration of Helsinki and was approved by the ethics
committees of each institute.
DAA treatment
The DAA regimens were daclatasvir/asnaprevir (n = 130, 41.7%), sofosbuvir/ledipasvir (n = 116, 37.2%), sofosbuvir/
ribavirin (n = 61, 19.6%) and ombitasvir/paritaprevir/ritonavir (n = 5, 1.6%). We assessed the response to DAA
treatment based on the presence of HCV-RNA at 12 weeks after the end of the treatment. Patients negative for
HCV-RNA at this time were considered to have achieved a sustained virological response (SVR12). The presence of
HCV-RNA was examined using real-time polymerase chain reaction.
Follow-up
After DAA treatment, the patients were assessed every three months by US, triple-phase CT, or MRI. The diagnosis
of HCC recurrence was confirmed via typical HCC imaging patterns obtained by angiography, CT, MRI and US.
The criteria for the imaging-based diagnosis of HCC have been described in previous reports: hyper-attenuation
at the hepatic arterial phase, hypo-attenuation at the portal venous phase in triple-phase CT or MRI and tumor
staining on angiography, or hyper-enhancement in the arterial phase and hypo-enhancement in the portal venous
and late phases on contrast-enhanced US [17,18] .
Statistical analyses
The clinical characteristics of the patients were obtained at the initial treatment for HCC and before and after DAA
treatment. The data collected included the age, gender, platelet count, albumin (ALB), total bilirubin (T.Bil), aspartate
aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGTP), prothrombin
time-international normalized ratio, Child-Pugh grade, AFP, des-γ-carboxy prothrombin (DCP), lens culinaris
agglutinin-reactive AFP-L3, fibrosis (FIB-4) index, HCV genotype, amount of HCV-RNA, history of HCC treatment,
number of tumors, maximum diameter of the tumor, date of HCC recurrence, treatment methods and survival.
Patients without recurrence were censored at the last visit or on the date of death. We calculated the annual HCC
recurrence rate after DAA therapy and examined the risk factors for recurrence using Cox proportional hazard models.
Variables with a P value of ≤ 0.1 in a univariate analysis were incorporated into a multivariate analysis by stepwise
selection. The recurrence rate and overall survival rate after DAA therapy were calculated using the Kaplan-Meier
method and evaluated with a log-rank test. P values of < 0.05 were considered to indicate statistical significance. All
analyses were performed using the JMP Pro 14.1.0 software package (SAS Institute, Inc., Cary, NC, USA).