Page 99 - Read Online
P. 99
Page 8 of 16 Bhatia et al. Hepatoma Res 2018;4:9 I http://dx.doi.org/10.20517/2394-5079.2018.04
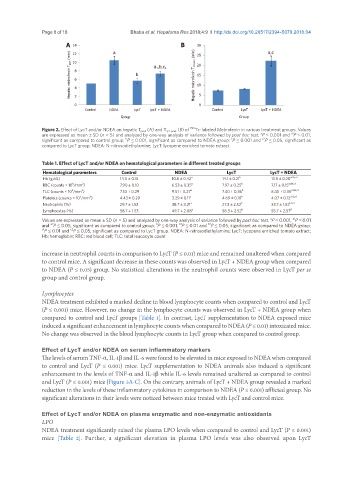
A 14 a B 30 a,c
Hepatic mebrofenin T peak (min) 10 8 6 4 b a 1 ,b,c 2 Hepatic mebrofenin T 1/2 peak (min) 20
12
25
15
10
0 2 5 0
Control NDEA LycT LycT + NDEA Control LycT LycT + NDEA
Group Group
Figure 2. Effect of LycT and/or NDEA on hepatic T peak (A) and T 1/2 peak (B) of 99m Tc-labeled Mebrofenin in various treatment groups. Values
a
are expressed as mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by post hoc test. P ≤ 0.001 and P ≤ 0.01,
a1
c2
significant as compared to control group; P ≤ 0.001, significant as compared to NDEA group; P ≤ 0.001 and P ≤ 0.05, significant as
b
c
compared to LycT group. NDEA: N-nitrosodiethylamine; LycT: lycopene enriched tomato extract
Table 1. Effect of LycT and/or NDEA on hematological parameters in different treated groups
Hematological parameters Control NDEA LycT LycT + NDEA
Hb (g/dL) 14.0 ± 0.15 10.6 ± 0.42 a 14.1 ± 0.21 b 13.5 ± 0.20 a2,b,c2
RBC (counts × 10 /mm ) 7.90 ± 0.10 6.53 ± 0.35 a 7.97 ± 0.25 b 7.17 ± 0.15 a1,b1,c1
6
3
3
3
TLC (counts × 10 /mm ) 7.53 ± 0.29 9.51 ± 0.27 a 7.40 ± 0.36 b 8.45 ± 0.30 a1,b1,c1
5
3
Platelets (counts × 10 /mm ) 4.43 ± 0.29 3.29 ± 0.11 a 4.69 ± 0.18 b 4.07 ± 0.12 a2,b,c1
Neutrophils (%) 29.7 ± 1.53 38.7 ± 3.21 a 27.3 ± 2.52 b 33.7 ± 1.52 b2,c1
Lymphocytes (%) 58.7 ± 1.53 49.7 ± 2.08 a 58.3 ± 2.52 b 55.7 ± 2.51 b1
a
a1
Values are expressed as mean ± SD (n = 5) and analyzed by one-way analysis of variance followed by post hoc test. P ≤ 0.001, P ≤ 0.01
a2
b1
b
b2
and P ≤ 0.05, significant as compared to control group; P ≤ 0.001, P ≤ 0.01 and P ≤ 0.05, significant as compared to NDEA group;
c1 c2
P ≤ 0.01 and P ≤ 0.05, significant as compared to LycT group. NDEA: N-nitrosodiethylamine; LycT: lycopene enriched tomato extract;
Hb: hemoglobin; RBC: red blood cell; TLC: total leucocyte count
increase in neutrophil counts in comparison to LycT (P ≤ 0.01) mice and remained unaltered when compared
to control mice. A significant decrease in these counts was observed in LycT + NDEA group when compared
to NDEA (P ≤ 0.05) group. No statistical alterations in the neutrophil counts were observed in LycT per se
group and control group.
Lymphocytes
NDEA treatment exhibited a marked decline in blood lymphocyte counts when compared to control and LycT
(P ≤ 0.001) mice. However, no change in the lymphocyte counts was observed in LycT + NDEA group when
compared to control and LycT groups [Table 1]. In contrast, LycT supplementation to NDEA exposed mice
induced a significant enhancement in lymphocyte counts when compared to NDEA (P ≤ 0.01) intoxicated mice.
No change was observed in the blood lymphocyte counts in LycT group when compared to control group.
Effect of LycT and/or NDEA on serum inflammatory markers
The levels of serum TNF-α, IL-1β and IL-6 were found to be elevated in mice exposed to NDEA when compared
to control and LycT (P ≤ 0.001) mice. LycT supplementation to NDEA animals also induced a significant
enhancement in the levels of TNF-α and IL-1β while IL-6 levels remained unaltered as compared to control
and LycT (P ≤ 0.001) mice [Figure 3A-C]. On the contrary, animals of LycT + NDEA group revealed a marked
reduction in the levels of these inflammatory cytokines in comparison to NDEA (P ≤ 0.001) afflicted group. No
significant alterations in their levels were noticed between mice treated with LycT and control mice.
Effect of LycT and/or NDEA on plasma enzymatic and non-enzymatic antioxidants
LPO
NDEA treatment significantly raised the plasma LPO levels when compared to control and LycT (P ≤ 0.001)
mice [Table 2]. Further, a significant elevation in plasma LPO levels was also observed upon LycT