Page 64 - Read Online
P. 64
Hung et al. Attenuation of liver stiffness by sorafenib
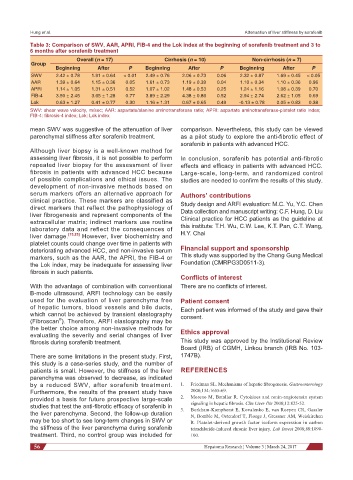
Table 3: Comparison of SWV, AAR, APRI, FIB-4 and the Lok index at the beginning of sorafenib treatment and 3 to
6 months after sorafenib treatment
Overall (n = 17) Cirrhosis (n = 10) Non-cirrhosis (n = 7)
Group
Beginning After P Beginning After P Beginning After P
SWV 2.42 ± 0.78 1.91 ± 0.64 < 0.01 2.49 ± 0.76 2.06 ± 0.73 0.06 2.32 ± 0.87 1.69 ± 0.45 < 0.05
AAR 1.39 ± 0.64 1.15 ± 0.36 0.05 1.61 ± 0.73 1.19 ± 0.39 0.04 1.10 ± 0.34 1.10 ± 0.36 0.96
APRI 1.14 ± 1.05 1.31 ± 0.51 0.52 1.07 ± 1.02 1.48 ± 0.53 0.25 1.24 ± 1.16 1.08 ± 0.39 0.70
FIB-4 3.50 ± 2.45 3.65 ± 1.28 0.77 3.89 ± 2.29 4.38 ± 0.86 0.52 2.94 ± 2.74 2.62 ± 1.05 0.69
Lok 0.63 ± 1.27 0.41 ± 0.77 0.30 1.16 ± 1.31 0.67 ± 0.65 0.49 -0.13 ± 0.78 0.05 ± 0.83 0.38
SWV: shear wave velocity, m/sec; AAR: aspartate/alanine aminotransferase ratio; APRI: aspartate aminotransferase-platelet ratio index;
FIB-4: fibrosis-4 index; Lok: Lok index
mean SWV was suggestive of the attenuation of liver comparison. Nevertheless, this study can be viewed
parenchymal stiffness after sorafenib treatment. as a pilot study to explore the anti-fibrotic effect of
sorafenib in patients with advanced HCC.
Although liver biopsy is a well-known method for
assessing liver fibrosis, it is not possible to perform In conclusion, sorafenib has potential anti-fibrotic
repeated liver biopsy for the assessment of liver effects and efficacy in patients with advanced HCC.
fibrosis in patients with advanced HCC because Large-scale, long-term, and randomized control
of possible complications and ethical issues. The studies are needed to confirm the results of this study.
development of non-invasive methods based on
serum markers offers an alternative approach for Authors’ contributions
clinical practice. These markers are classified as Study design and ARFI evaluation: M.C. Yu, Y.C. Chen
direct markers that reflect the pathophysiology of Data collection and manuscript writing: C.F. Hung, D. Liu
liver fibrogenesis and represent components of the
extracellular matrix; indirect markers use routine Clinical practice for HCC patients as the guideline at
laboratory data and reflect the consequences of this institute: T.H. Wu, C.W. Lee, K.T. Pan, C.T. Wang,
liver damage. [15,25] However, liver biochemistry and H.Y. Chai
platelet counts could change over time in patients with
deteriorating advanced HCC, and non-invasive serum Financial support and sponsorship
markers, such as the AAR, the APRI, the FIB-4 or This study was supported by the Chang Gung Medical
the Lok index, may be inadequate for assessing liver Foundation (CMRPG3D0511-3).
fibrosis in such patients.
Conflicts of interest
With the advantage of combination with conventional There are no conflicts of interest.
B-mode ultrasound, ARFI technology can be easily
used for the evaluation of liver parenchyma free Patient consent
of hepatic tumors, blood vessels and bile ducts, Each patient was informed of the study and gave their
which cannot be achieved by transient elastography consent.
®
(Fibroscan ). Therefore, ARFI elastography may be
the better choice among non-invasive methods for Ethics approval
evaluating the severity and serial changes of liver
fibrosis during sorafenib treatment. This study was approved by the Institutional Review
Board (IRB) of CGMH, Linkou branch (IRB No. 103-
There are some limitations in the present study. First, 1747B).
this study is a case-series study, and the number of
patients is small. However, the stiffness of the liver REFERENCES
parenchyma was observed to decrease, as indicated
by a reduced SWV, after sorafenib treatment. 1. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology
Furthermore, the results of the present study have 2008;134:1655-69.
provided a basis for future prospective large-scale 2. Moreno M, Bataller R. Cytokines and renin-angiotensin system
studies that test the anti-fibrotic efficacy of sorafenib in 3. signaling in hepatic fibrosis. Clin Liver Dis 2008;12:825-52.
Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler
the liver parenchyma. Second, the follow-up duration N, Bomble M, Ostendorf T, Floege J, Gressner AM, Weiskirchen
may be too short to see long-term changes in SWV or R. Platelet-derived growth factor isoform expression in carbon
the stiffness of the liver parenchyma during sorafenib tetrachloride-induced chronic liver injury. Lab Invest 2008;88:1090-
treatment. Third, no control group was included for 100.
56 Hepatoma Research ¦ Volume 3 ¦ March 24, 2017