Page 27 - Read Online
P. 27
Selvakumar et al. Multikinase inhibitors as neo-adjuvants in HCC
liver transplantation various neoadjuvant modalities did not have any radiological signs of viable disease
have evolved over years to make inoperable patients the plan for palliative radiotherapy was cancelled.
into operable with equivalent survival rates. TACE, After assessment for living-donor liver transplantation
RFA and EBRT have been employed as neoadjuvant (LDLT) and after discussion of the case in the liver
modalities to reduce the tumor burden. There are transplant meeting, it was decided to do LDLT.
resolution chest tomographies (RCTs) going on to
assess the effect of neoadjuvant role of TACE with or On admission, investigations revealed Hb 12.10, TLC
without sorafenib. Our case reports give a different 5,860/cu mm, platelet count 198,000/cu mm, prothrombin
perspective to these ongoing studies. One case was time/international normalized ratio (PT/INR) 9.40/0.90,
sorafenib without hepatic artery occlusion and the urea 25 mg/dL, creatinine 0.70 mg/dL, serum bilirubin
other one with hepatic artery occlusion. 0.60 mg/dL, albumin 3.60 mg/dL. Anti HCV was
reactive and HBsAg & HIV were non-reactive. Serum
CASE REPORT AFP was 3.52 IU/mL. Urine protein/creatinine ratio
was 0.24. PET-CT liver showed cirrhotic liver with a
Case 1 small right lobe and multiple SOL’s in the residual right
A 54-year-old gentleman, a business man from lobe and tumor thrombus in right portal veins and main
Islamabad, was diagnosed with hepatitis C virus portal veins/left portal veins junction as described, mild
(HCV) infection in 2003 when he was worked up ascites. Magnetic resonance imaging upper abdomen
for generalized weakness. For which he received showed liver cirrhosis, multiple masses in both lobes
26 injections of peg-interferon over 3 months of liver (right > left) with tumor thrombus in right, left
and achieved sustained viral response (SVR). and main portal vein near portal bifurcation suggestive
He remained relatively asymptomatic till 2015. In of HCC, bland thrombus in remaining portal vein, no
September 2015, he developed right upper quadrant significant abdominal lymphadenopathy or ascites
pain associated with significant loss of weight. In is seen. High RCT showed no scan evidence of
October 2015, he was diagnosed with HCC in the right pulmonary metastasis. 2D Echo showed pulmonary
lobe with portal vein tumor thrombosis (PVTT) and artery systolic pressure 22, CVP 5, EF 60% and
encasement of right hepatic vein and middle hepatic dobutamine stress echocardiography was negative.
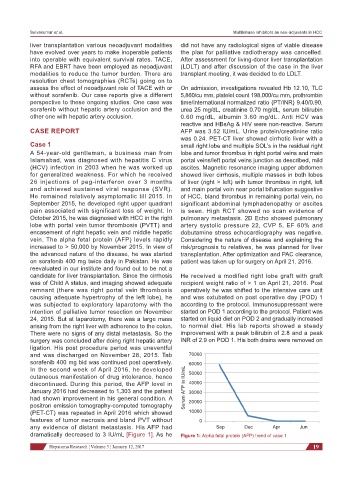
vein. The alpha fetal protein (AFP) levels rapidly Considering the nature of disease and explaining the
increased to > 50,000 by November 2015. In view of risk/prognosis to relatives, he was planned for liver
the advanced nature of the disease, he was started transplantation. After optimization and PAC clearance,
on sorafenib 400 mg twice daily in Pakistan. He was patient was taken up for surgery on April 21, 2016.
reevaluated in our institute and found out to be not a
candidate for liver transplantation. Since the cirrhosis He received a modified right lobe graft with graft
was of Child A status, and imaging showed adequate recipient weight ratio of > 1 on April 21, 2016. Post
remnant (there was right portal vein thrombosis operatively he was shifted to the intensive care unit
causing adequate hypertrophy of the left lobe), he and was extubated on post operative day (POD) 1
was subjected to exploratory laparotomy with the according to the protocol. Immunosuppressant were
intention of palliative tumor resection on November started on POD 1 according to the protocol. Patient was
24, 2015. But at laparotomy, there was a large mass started on liquid diet on POD 2 and gradually increased
arising from the right liver with adherence to the colon. to normal diet. His lab reports showed a steady
There were no signs of any distal metastasis. So the improvement with a peak bilirubin of 2.8 and a peak
surgery was concluded after doing right hepatic artery INR of 2.9 on POD 1. His both drains were removed on
ligation. His post procedure period was uneventful
and was discharged on November 28, 2015. Tab 70000
sorafenib 400 mg bid was continued post operatively.
In the second week of April 2016, he developed
cutaneous manifestation of drug intolerance, hence 50000
discontinued. During this period, the AFP level in Serum AFP in IU/mL 60000
40000
January 2016 had decreased to 1,303 and the patient 30000
had shown improvement in his general condition. A 20000
positron emission tomography-computed tomography
(PET-CT) was repeated in April 2016 which showed 10000
features of tumor necrosis and bland PVT without 0
any evidence of distant metastasis. His AFP had Sep Dec Apr Jun
dramatically decreased to 3 IU/mL [Figure 1]. As he Figure 1: Alpha fetal protein (AFP) trend of case 1
Hepatoma Research ¦ Volume 3 ¦ January 12, 2017 19