Page 234 - Read Online
P. 234
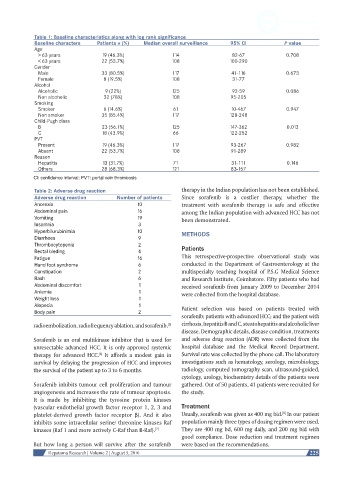
Table 1: Baseline characteristics along with log rank significance
Baseline characters Patients n (%) Median overall surveillance 95% CI P value
Age
> 63 years 19 (46.3%) 114 82-67 0.708
< 63 years 22 (53.7%) 108 100-290
Gender
Male 33 (80.5%) 117 41-116 0.673
Female 8 (19.5%) 108 31-77
Alcohol
Alcoholic 9 (22%) 125 92-59 0.086
Non alcoholic 32 (78%) 108 95-205
Smoking
Smoker 6 (14.6%) 61 10-467 0.947
Non smoker 35 (85.4%) 117 128-248
Child-Pugh class
B 23 (56.1%) 125 147-362 0.013
C 18 (43.9%) 66 122-252
PVT
Present 19 (46.3%) 117 93-267 0.982
Absent 22 (53.7%) 108 91-289
Reason
Hepatitis 13 (31.7%) 71 31-111 0.146
Others 28 (68.3%) 121 83-157
CI: confidence interval; PVT: portal vein thrombosis
Table 2: Adverse drug reaction therapy in the Indian population has not been established.
Adverse drug reaction Number of patients Since sorafenib is a costlier therapy, whether the
Anorexia 10 treatment with sorafenib therapy is safe and effective
Abdominal pain 16 among the Indian population with advanced HCC has not
Vomiting 19 been demonstrated.
Insomnia 3
Hyperbilurubinimia 10 METHODS
Diarrhoea 9
Thrombocytopenia 2
Rectal bleding 4 Patients
Fatigue 16 This retrospective-prospective observational study was
Hand foot syndrome 6 conducted in the Department of Gastroenterology at the
Constipation 2 multispecialty teaching hospital of P.S.G Medical Science
Rash 6 and Research Institute, Coimbatore. Fifty patients who had
Abdominal discomfort 1 received sorafenib from January 2009 to December 2014
Aniemia 1 were collected from the hospital database.
Weight loss 1
Alopecia 1 Patient selection was based on patients treated with
Body pain 2
sorafenib; patients with advanced HCC; and the patient with
radioembolization, radiofrequency ablation, and sorafenib. [5] cirrhosis, hepatitis B and C, steatohepatitis and alcoholic liver
disease. Demographic details, disease condition, treatments
Sorafenib is an oral multikinase inhibitor that is used for and adverse drug reaction (ADR) were collected from the
unresectable advanced HCC. It is only approved systemic hospital database and the Medical Record Department.
therapy for advanced HCC. It affords a modest gain in Survival rate was collected by the phone call. The laboratory
[6]
survival by delaying the progression of HCC and improves investigations such as hematology, serology, microbiology,
the survival of the patient up to 3 to 6 months. radiology, computed tomography scan, ultrasound-guided,
cytology, urology, biochemistry details of the patients were
Sorafenib inhibits tumour cell proliferation and tumour gathered. Out of 50 patients, 41 patients were recruited for
angiogenesis and increases the rate of tumour apoptosis. the study.
It is made by inhibiting the tyrosine protein kinases
(vascular endothelial growth factor receptor 1, 2, 3 and Treatment
[8]
platelet-derived growth factor receptor β). And it also Usually, sorafenib was given as 400 mg bid. In our patient
inhibits some intracellular serine/ threonine kinases Raf population mainly three types of dosing regimen were used.
kinases (Raf 1 and more actively C-Raf than B-Raf). [7] They are 400 mg bd, 600 mg daily, and 200 mg bid with
good compliance. Dose reduction and treatment regimen
But how long a person will survive after the sorafenib were based on the recommendations.
Hepatoma Research | Volume 2 | August 5, 2016 225