Page 119 - Read Online
P. 119
[16]
for development of HCC. Thus, telomerase has been
recognized as a relevant factor in distinguishing cancer from
normal cells and is a very promising target for anticancer
therapy. [17]
STRUCTURE AND WORKING MECHANISM OF
TELOMERASE
Telomerase is a unique reverse transcriptase, the core of
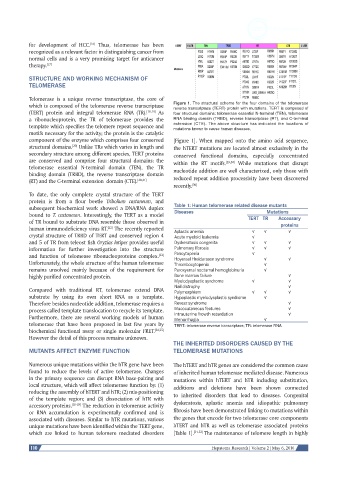
which is composed of the telomerase reverse transcriptase Figure 1. The structural scheme for the four domains of the telomerase
reverse transcriptase (TERT) protein with mutations. TERT is composed of
(TERT) protein and integral telomerase RNA (TR). [18,19] As four structural domains: telomerase essential N-terminal (TEN), telomerase
a ribonucleoprotein, the TR of telomerase provides the RNA binding domain (TRBD), reverse transcriptase (RT), and C-terminal
template which specifies the telomere repeat sequence and extension (CTE). The above structure has indicated the locations of
mutations known to cause human diseases.
motifs necessary for the activity; the protein is the catalytic
component of the enzyme which comprises four conserved [Figure 1]. When mapped onto the amino acid sequence,
structural domains. Unlike TRs which varies in length and the hTERT mutations are located almost exclusively in the
[20]
secondary structure among different species, TERT proteins conserved functional domains, especially concentrated
are conserved and comprise four structural domains: the within the RT motifs. [29,30] While mutations that disrupt
telomerase essential N-terminal domain (TEN), the TR nucleotide addition are well characterized, only those with
binding domain (TRBD), the reverse transcriptase domain
(RT) and the C-terminal extension domain (CTE). [20,21] reduced repeat addition processivity have been discovered
recently. [30]
To date, the only complete crystal structure of the TERT
protein is from a flour beetle Tribolium castaneum, and
subsequent biochemical work showed a DNA/RNA duplex Table 1: Human telomerase related disease mutants
bound to T. castaneum. Interestingly, the TERT as a model Diseases Mutations
of TR bound to substrate DNA resemble those observed in TERT TR Accessary
human immunodeficiency virus RT. The recently reported Aplastic anemia √ √ proteins
[22]
√
crystal structure of TRBD of TERT and conserved region 4 Acute myeloid leukemia √
and 5 of TR from teleost fish Oryzias latipes provides useful Dyskeratosis congenita √ √ √
information for further investigation into the structure Pulmonary fibrosis √ √ √
[23]
and function of telomease ribonucleoproteins complex. Pancytopenia √ √ √
Hoyeraal Hreidarsson syndrome
Unfortunately, the whole structure of the human telomerase Thrombocytopenia √
remains unsolved mainly because of the requirement for Paroxysmal nocturnal hemoglobinuria √
highly purified concentrated protein. Bone marrow failure √
Myelodysplastic syndrome √ √
Nail distrophy √
Compared with traditional RT, telomerase extend DNA Polymorphism √ √ √
substrate by using its own short RNA as a template. Hypoplastic myelodysplastic syndrome √
Therefore besides nucleotide addition, telomerase requires a Revesz syndrome √
process called template translocation to recycle its template. Mucocutaneous features √ √
Furthermore, there are several working models of human Intrauterine frowth retardation √
Menorrhagia
telomerase that have been proposed in last few years by TERT: telomerase reverse transcriptase; TR: telomerase RNA
biochemical functional assay or single molecular FRET. [24,25]
However the detail of this process remains unknown.
THE INHERITED DISORDERS CAUSED BY THE
MUTANTS AFFECT ENZYME FUNCTION TELOMERASE MUTATIONS
Numerous unique mutations within the hTR gene have been The hTERT and hTR genes are considered the common cause
found to reduce the levels of active telomerase. Changes of inherited human telomerase mediated disease. Numerous
in the primary sequence can disrupt RNA base-pairing and mutations within hTERT and hTR including substitution,
local structure, which will affect telomerase function by: (1) additions and deletions have been shown connected
reducing the assembly of hTERT and hTR; (2) mis-positioning to inherited disorders that lead to diseases. Congenital
of the template region; and (3) dissociation of hTR with
accessory proteins. [26-28] The reduction in telomerase activity dyskeratosis, aplastic anemia and idiopathic pulmonary
or RNA accumulation is experimentally confirmed and is fibrosis have been demonstrated linking to mutations within
associated with diseases. Similar to hTR mutations, various the genes that encode for two telomerase core components
unique mutations have been identified within the TERT gene, hTERT and hTR as well as telomerase associated proteins
which are linked to human telomere mediated disorders [Table 1]. [31-33] The maintenance of telomere length in highly
110 Hepatoma Research | Volume 2 | May 6, 2016