Page 67 - Read Online
P. 67
note that even the adverse events resulting in permanent The first non-Asian study investigating the use of sorafenib
discontinuation of the drug were similar in both groups. [13] in a large cohort of elderly patients was published in 2013.
Di Costanzo et al. [14] analyzed a cohort of consecutive
The impact of age on the effects of sorafenib in clinical patients not eligible for surgery or loco-regional treatment,
practice was also examined in different single and multicenter with Child-Pugh score ≤ 7, treated with sorafenib.
experiences [14-20] and discussed in a few reviews. [21,22] A table Clinical outcomes and treatment-related adverse events
summarizing the efficacy and the safety of older and younger were compared between younger (< 70 years) and
patients treated with sorafenib reported in most papers so older (≥ 70 years) patients. Overall, 150 patients (90 in the
far published are reported [Table 1]. younger and 60 in the older group) were evaluated. The study
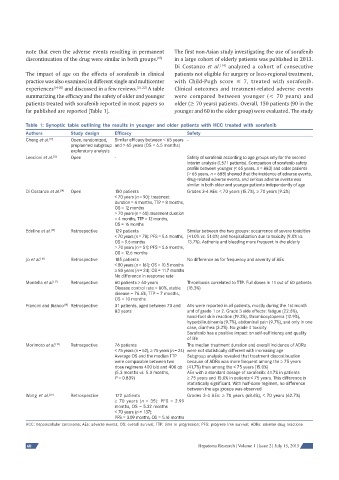
Table 1: Synoptic table outlining the results in younger and older patients with HCC treated with sorafenib
Authors Study design Effi cacy Safety
Cheng et al. [12] Open, randomized, Similar effi cacy between < 65 years -
preplanned subgroup and > 65 years (OS = 6.5 months)
exploratory analysis
Lencioni et al. [13] Open - Safety of sorafenib according to age groups only for the second
interim analysis (1,571 patients). Comparison of sorafenib safety
profi le between younger (< 65 years, n = 883) and older patients
(≥ 65 years, n = 688) showed that the incidence of adverse events,
drug-related adverse events, and serious adverse events was
similar in both older and younger patients independently of age
Di Costanzo et al. [14] Open 150 patients Grades 3-4 AEs: < 70 years (15.7%), ≥ 70 years (9.2%)
< 70 years (n = 90): treatment
duration = 4 months, TTP = 8 months,
OS = 12 months
≥ 70 years (n = 60): treatment duration
= 4 months, TTP = 12 months,
OS = 16 months
Edeline et al. [15] Retrospective 129 patients Similar between the two groups: occurrence of severe toxicities
< 70 years (n = 78): PFS = 5.6 months, (41.0% vs. 51.0%) and hospitalization due to toxicity (9.0% vs.
OS = 9.6 months 13.7%). Asthenia and bleeding more frequent in the elderly
≥ 70 years (n = 51): PFS = 5.6 months,
OS = 12.6 months
Jo et al. [16] Retrospective 185 patients No difference as for frequency and severity of AEs
< 80 years (n = 161): OS = 10.5 months
≥ 80 years (n = 24): OS = 11.7 months
No difference in response rate
Montella et al. [17] Retrospective 60 patients > 60 years Thrombosis correlated to TTP. Full doses in 11 out of 60 patients
Disease control rate = 80%, stable (18.3%)
disease = 76.6%, TTP = 7 months,
OS = 10 months
Francini and Bianco [18] Retrospective 31 patients, aged between 70 and AEs were reported in all patients, mostly during the 1st month
83 years and of grade 1 or 2. Grade 3 side effects: fatigue (22.6%),
hand-foot skin reaction (19.3%), thrombocytopenia (12.9%),
hyperbilirubinemia (9.7%), abdominal pain (9.7%), and only in one
case, diarrhea (3.2%). No grade 4 toxicity
Sorafenib has a positive impact on self-suffi ciency and quality
of life
Morimoto et al. [19] Retrospective 76 patients The median treatment duration and overall incidence of ADRs
< 75 years (n = 52), ≥ 75 years (n = 24) were not statistically different with increasing age
Average OS and the median TTP Subgroup analysis revealed that treatment discontinuation
were comparable between two because of ADRs was more frequent among the ≥ 75 years
dose regimens 400 bid and 400 qb (41.7%) than among the < 75 years (15.0%)
(5.3 months vs. 5.0 months, AEs with a standard dosage of sorafenib: 41.7% in patients
P = 0.839) ≥ 75 years and 15.0% in patients < 75 years. This difference is
statistically signifi cant. With half-dose regimen, no difference
between the age groups was observed
Wong et al. [20] Retrospective 172 patients Grades 3-4 AEs: ≥ 70 years (68.6%), < 70 years (62.7%)
≥ 70 years (n = 35): PFS = 2.99
months, OS = 5.32 months
< 70 years (n = 137):
PFS = 3.09 months, OS = 5.16 months
HCC: hepatocellular carcinoma; AEs: adverse events; OS: overall survival; TTP: time to progression; PFS: progress free survival; ADRs: adverse drug reactions
60 Hepatoma Research | Volume 1 | Issue 2 | July 15, 2015