Page 42 - Read Online
P. 42
Anugwom et al. Hepatoma Res 2022;8:7 https://dx.doi.org/10.20517/2394-5079.2021.123 Page 3 of 13
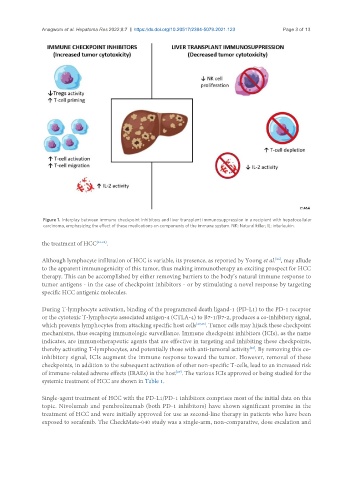
Figure 1. Interplay between immune checkpoint inhibitors and liver transplant immunosuppression in a recipient with hepatocellular
carcinoma, emphasizing the effect of these medications on components of the immune system. NK: Natural killer; IL: interleukin.
the treatment of HCC [22-25] .
[26]
Although lymphocyte infiltration of HCC is variable, its presence, as reported by Yoong et al. , may allude
to the apparent immunogenicity of this tumor, thus making immunotherapy an exciting prospect for HCC
therapy. This can be accomplished by either removing barriers to the body’s natural immune response to
tumor antigens - in the case of checkpoint inhibitors - or by stimulating a novel response by targeting
specific HCC antigenic molecules.
During T-lymphocyte activation, binding of the programmed death ligand-1 (PD-L1) to the PD-1 receptor
or the cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) to B7-1/B7-2, produces a co-inhibitory signal,
which prevents lymphocytes from attacking specific host cells [27,28] . Tumor cells may hijack these checkpoint
mechanisms, thus escaping immunologic surveillance. Immune checkpoint inhibitors (ICIs), as the name
indicates, are immunotherapeutic agents that are effective in targeting and inhibiting these checkpoints,
thereby activating T-lymphocytes, and potentially those with anti-tumoral activity . By removing this co-
[28]
inhibitory signal, ICIs augment the immune response toward the tumor. However, removal of these
checkpoints, in addition to the subsequent activation of other non-specific T-cells, lead to an increased risk
of immune-related adverse effects (IRAEs) in the host . The various ICIs approved or being studied for the
[29]
systemic treatment of HCC are shown in Table 1.
Single-agent treatment of HCC with the PD-L1/PD-1 inhibitors comprises most of the initial data on this
topic. Nivolumab and pembrolizumab (both PD-1 inhibitors) have shown significant promise in the
treatment of HCC and were initially approved for use as second-line therapy in patients who have been
exposed to sorafenib. The CheckMate-040 study was a single-arm, non-comparative, dose escalation and