Page 43 - Read Online
P. 43
Page 4 of 13 Anugwom et al. Hepatoma Res 2022;8:7 https://dx.doi.org/10.20517/2394-5079.2021.123
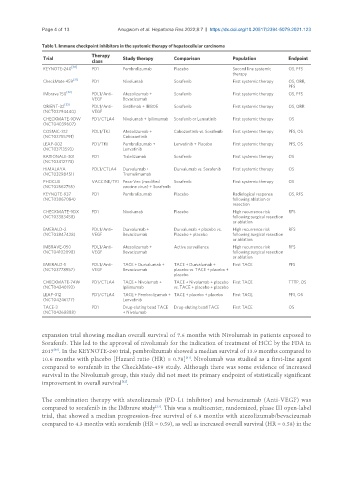
Table 1. Immune checkpoint inhibitors in the systemic therapy of hepatocellular carcinoma
Therapy
Trial Study therapy Comparison Population Endpoint
class
[30]
KEYNOTE-240 PD1 Pembrolizumab Placebo Second line systemic OS, PFS
therapy
[31]
CheckMate 459 PD1 Nivolumab Sorafenib First systemic therapy OS, ORR,
PFS
[32]
IMbrave 150 PDL1/Anti- Atezolizumab + Sorafenib First systemic therapy OS, PFS
VEGF Bevacizumab
ORIENT-32 [33] PDL1/Anti- Sintilimab + IBI305 Sorafenib First systemic therapy OS, ORR
(NCT03794440) VEGF
CHECKMATE-9DW PD1/CTLA4 Nivolumab + Ipilimumab Sorafenib or Lenvatinib First systemic therapy OS
(NCT04039607)
COSMIC-312 PDL1/TKI Atezolizumab + Cabozantinib vs. Sorafenib First systemic therapy PFS, OS
(NCT03755791) Cabozantinib
LEAP-002 PD1/TKI Pembrolizumab + Lenvatinib + Placebo First systemic therapy PFS, OS
(NCT03713593) Lenvatinib
RATIONALE-301 PD1 Tislelizumab Sorafenib First systemic therapy OS
(NCT03412773)
HIMALAYA PDL1/CTLA4 Durvalumab + Durvalumab vs. Sorafenib First systemic therapy OS
(NCT03298451) Tremelimumab
PHOCUS VACCINE/TKI Pexa-Vec (modified Sorafenib First systemic therapy OS
(NCT02562755) vaccine virus) + Sorafenib
KEYNOTE-937 PD1 Pembrolizumab Placebo Radiological response OS, RFS
(NCT03867084) following ablation or
resection
CHECKMATE-9DX PD1 Nivolumab Placebo High recurrence risk RFS
(NCT03383458) following surgical resection
or ablation
EMERALD-2 PDL1/Anti- Durvalumab + Durvalumab + placebo vs. High recurrence risk RFS
(NCT03847428) VEGF Bevacizumab Placebo + placebo following surgical resection
or ablation
IMBRAVE-050 PDL1/Anti- Atezolizumab + Active surveillance High recurrence risk RFS
(NCT04102098) VEGF Bevacizumab following surgical resection
or ablation
EMERALD-1 PDL1/Anti- TACE + Durvalumab + TACE + Durvalumab + First TACE PFS
(NCT03778957) VEGF Bevacizumab placebo vs. TACE + placebo +
placebo
CHECKMATE-74W PD1/CTLA4 TACE + Nivolumab + TACE + Nivolumab + placebo First TACE TTTP, OS
(NCT04340193) Ipilimumab vs. TACE + placebo + placebo
LEAP-012 PD1/CTLA4 TACE + Pembrolizumab + TACE + placebo + placebo First TACE PFS, OS
(NCT04246177) Lenvatinib
TACE-3 PD1 Drug-eluting bead TACE Drug-eluting bead TACE First TACE OS
(NCT04268888) + Nivolumab
expansion trial showing median overall survival of 7.6 months with Nivolumab in patients exposed to
Sorafenib. This led to the approval of nivolumab for the indication of treatment of HCC by the FDA in
2017 . In the KEYNOTE-240 trial, pembrolizumab showed a median survival of 13.9 months compared to
[30]
[31]
10.6 months with placebo [Hazard ratio (HR) = 0.78] . Nivolumab was studied as a first-line agent
compared to sorafenib in the CheckMate-459 study. Although there was some evidence of increased
survival in the Nivolumab group, this study did not meet its primary endpoint of statistically significant
improvement in overall survival .
[32]
The combination therapy with atezolizumab (PD-L1 inhibitor) and bevacizumab (Anti-VEGF) was
compared to sorafenib in the IMbrave study . This was a multicenter, randomized, phase III open-label
[33]
trial, that showed a median progression-free survival of 6.8 months with atezolizumab/bevacizumab
compared to 4.3 months with sorafenib (HR = 0.59), as well as increased overall survival (HR = 0.58) in the