Page 20 - Read Online
P. 20
Page 8 of 14 Daniele et al. Hepatoma Res 2021;7:61 https://dx.doi.org/10.20517/2394-5079.2021.58
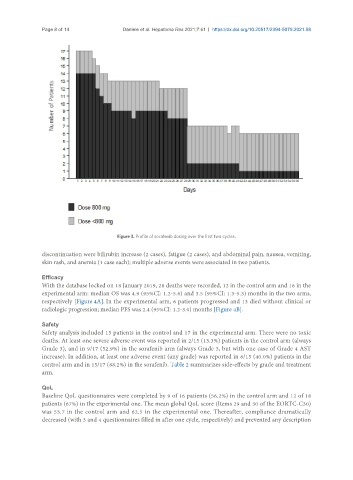
Figure 3. Profile of sorafenib dosing over the first two cycles.
discontinuation were bilirubin increase (2 cases), fatigue (2 cases), and abdominal pain, nausea, vomiting,
skin rash, and anemia (1 case each); multiple adverse events were associated in two patients.
Efficacy
With the database locked on 18 January 2019, 28 deaths were recorded, 12 in the control arm and 16 in the
experimental arm: median OS was 4.9 (95%CI: 1.2-5.6) and 3.5 (95%CI: 1.3-5.3) months in the two arms,
respectively [Figure 4A]. In the experimental arm, 6 patients progressed and 13 died without clinical or
radiologic progression; median PFS was 2.4 (95%CI: 1.2-3.0) months [Figure 4B].
Safety
Safety analysis included 15 patients in the control and 17 in the experimental arm. There were no toxic
deaths. At least one severe adverse event was reported in 2/15 (13.3%) patients in the control arm (always
Grade 3), and in 9/17 (52.9%) in the sorafenib arm (always Grade 3, but with one case of Grade 4 AST
increase). In addition, at least one adverse event (any grade) was reported in 6/15 (40.0%) patients in the
control arm and in 15/17 (88.2%) in the sorafenib. Table 2 summarizes side-effects by grade and treatment
arm.
QoL
Baseline QoL questionnaires were completed by 9 of 16 patients (56.2%) in the control arm and 12 of 18
patients (67%) in the experimental one. The mean global QoL score (Items 29 and 30 of the EORTC-C30)
was 53.7 in the control arm and 62.5 in the experimental one. Thereafter, compliance dramatically
decreased (with 3 and 4 questionnaires filled in after one cycle, respectively) and prevented any description