Page 16 - Read Online
P. 16
Page 4 of 14 Daniele et al. Hepatoma Res 2021;7:61 https://dx.doi.org/10.20517/2394-5079.2021.58
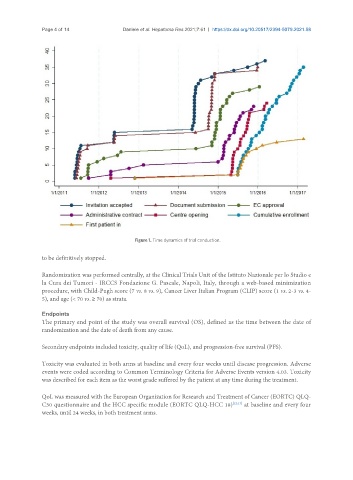
Figure 1. Time dynamics of trial conduction.
to be definitively stopped.
Randomization was performed centrally, at the Clinical Trials Unit of the Istituto Nazionale per lo Studio e
la Cura dei Tumori - IRCCS Fondazione G. Pascale, Napoli, Italy, through a web-based minimization
procedure, with Child-Pugh score (7 vs. 8 vs. 9), Cancer Liver Italian Program (CLIP) score (1 vs. 2-3 vs. 4-
5), and age (< 70 vs. ≥ 70) as strata.
Endpoints
The primary end point of the study was overall survival (OS), defined as the time between the date of
randomization and the date of death from any cause.
Secondary endpoints included toxicity, quality of life (QoL), and progression-free survival (PFS).
Toxicity was evaluated in both arms at baseline and every four weeks until disease progression. Adverse
events were coded according to Common Terminology Criteria for Adverse Events version 4.03. Toxicity
was described for each item as the worst grade suffered by the patient at any time during the treatment.
QoL was measured with the European Organization for Research and Treatment of Cancer (EORTC) QLQ-
C30 questionnaire and the HCC specific module (EORTC QLQ-HCC 18) [12,13] at baseline and every four
weeks, until 24 weeks, in both treatment arms.